Pulmocis Kit For Preparation Of Technetium Human Albumin Macroaggregates Injection
SUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE MEDICINAL PRODUCT
PULMOCIS 2 mg kit for radiopharmaceutical preparation.
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each vial contains 2 mg of human albumin as macroaggregates.
The radionuclide is not part of the kit.
The macroaggregates number per vial is ranging between 2 and 4 millions.
No macroaggregates has a size higher than 150 pm.
Not more than 10 of them have a size higher than 100pm.
Excipient(s) with known effect:
Each vial contains 3.5 mg of sodium.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Kit for radiopharmaceutical preparation White pellet.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
This medicinal product is for diagnostic use only.
After radiolabelling with sodium pertechnetate (99mTc) solution, the suspension of technetium(99mTc) -albumin macroaggregates (or 99mTc-MAA) obtained is indicated for: Pulmonary perfusion scintigraphy.
As secondary indication 99mTc-albumin macroaggregates may be used for venoscintigraphy.
4.2 Posology and method of administration
This medicinal product is intended for use in designated nuclear medicine facilities only, and should only be handled by authorised personnel.
Posology
Adults
The recommended activity administered to an adult weighing 70 kg varies between 37 and 185 MBq, The number of particles per administered dose must be in a range of 60 x 103 - 700 x 103.
Paediatric population
The use in children and adolescents has to be considered carefully, based upon clinical needs and assessing the risk/benefit ratio in this patient group. The activities to be administered to children and to adolescents should be a fraction of the adult activity and should be calculated according to the following equation:
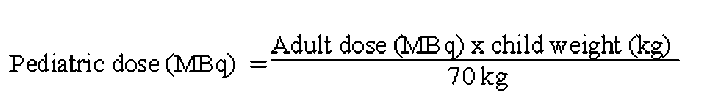
Although body weight is the more used factor on which to base the adjustment of the activity administered, in a limited number of cases the body surface area may be considered to be more appropriate.
Pediatric dose (MBq) =
Adult dose (MBq) x child surface (m2)
1.73
Method of administration Multidose use.
This medicinal product should be reconstituted before administration to the patient.
The radiolabelled solution must be administered by slow intravenous injection.
For instructions on extemporaneous preparation of the medicinal product before administration, see section 12.
For patient preparation, see section 4.4.
Image acquisition
The lung test can be started immediately after injection.
In pulmonary scintigraphy, images can be acquired in anterior, right/left oblique, right/left profile and posterior position.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 or to any of the components of the labelled radiopharmaceutical.
4.4 Special warnings and precautions for use
Potential for hypersensitivity or anaphylactic reactions
The possibility of hypersensitivity including serious, life-threatening, fatal anaphylactic/anaphylactoid reactions should always be considered. If hypersensitivity or anaphylactic reactions occur, the administration of the medicinal product must be discontinued immediately and intravenous treatment initiated, if necessary. To enable immediate action in emergencies, the necessary medicinal products and equipment such as endotracheal tube and ventilator must be immediately available.
Individual benefit/risk justification
For each patient, the radiation exposure must be justifiable by the likely benefit.
The activity administered should in every case be as low as reasonably achievable to obtain the required diagnostic information.
In patients with significant right to left cardiac shunt, or with respiratory failure complicating pulmonary hypertension, special care should be exercised when administering 99mTc albumin macroaggregates (MAA). In order to minimise the possibility of microembolism to the cerebral and renal circulations 99mTc-MAA should be given by slow intravenous injection and the number of particles reduced by up 50%.
Paediatric _ population
For information on the use in paediatric population, see sections 4.2.
Careful consideration of the indication is required since the effective dose per MBq is higher than in adults (see section 11).
Patient preparation
The patient should be well hydrated before the start of the examination and urged to void as often as possible during the first hours after the examination in order to reduce radiation.
Specific warnings
PULMOCIS contains human albumin.
Standard measures for preventing transmission of infections from pharmaceuticals made of human blood or plasma, include selection of donators, test of individual donators and plasma pools for finding specific infective agents, and effective manufacturing steps for inactivation/elimination of virus as a part of manufacturing process as well. In spite of that, the risk of transmission of infectious agents cannot be eliminated completely, as long as pharmaceuticals made of human blood or plasma are used. This also applies to new virus of unknown nature and other pathogens as well.
There are no reports of virus transmission in connection with albumin, made in accordance with specifications in Ph. Eur. and in accordance with routine processes.
It is strongly recommended that the product name and batch number are stated every time Pulmocis is given to a patient, in order to maintain a connection between the patient and the product’s batch number.
The syringe should be gently swirled immediately prior to the injection to homogenise the injectate. Blood should never be drawn into the syringe because that induces the formation of small clots.
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially ‘sodium-free’.
Precautions with respect to environmental hazard see section 6.6.
4.5 Interaction with other medicinal products and other forms of interaction
Changes in the biological distribution of 99mTc-MAA may be induced by different drugs.
- Pharmacologic interactions may be caused by chemotherapeutic agents, heparin and bronchodilators.
- Toxicological interactions may be caused by heroin, nitrofurantoin, busulfan, cyclophosphamide, bleomycin, methotrexate, methysergide.
- Pharmaceutical interactions may be caused by magnesium sulphate.
4.6 Fertility, pregnancy and lactation
Women of childbearing potential
When an administration of radiopharmaceuticals to a woman of childbearing potential is intended, it is important to determine whether or not she is pregnant. Any woman who has missed a period should be assumed to be pregnant until proven otherwise. If in doubt about her potential pregnancy (if the woman has missed a period, if the period is very irregular, etc.), alternative techniques not using ionising radiation (if there are any) should be offered to the patient.
Pregnancy:
Radionuclide procedures carried out on pregnant women also involve radiation doses to the foetus. Only essential investigations should therefore be carried out during pregnancy, when the likely benefit far exceeds the risk incurred by the mother and the foetus.
Breast feeding:
Before administering radiopharmaceuticals to a mother who is breastfeeding consideration should be given to the possibility of delaying the administration of radionuclide until the mother has ceased breastfeeding, and to what is the most appropriate choice of radiopharmaceutical has been made, bearing in mind the secretion of radioactivity in breast milk. If the administration is considered necessary, breast feeding should be interrupted for 12 hours and the expressed feeds discarded.
4.7 Effects on ability to drive and use machines
No studies on the effects on ability to drive and use machines have been performed.
4.8 Undesirable effects
For safety with respect to transmissible agents see section 4.4.
The frequencies of undesirable effects are defined as follows:
Very common (>1/10), common (>1/100 to <1/10), uncommon (>1/1,000 to <1/100), rare (>1/10,000 to <1/1,000), very rare (<1/10,000) and not known (cannot be estimated from the available data)
Immune system disorders
Frequency not known: Hypersensitivity-type reactions, including life-threatening anaphylaxis. Application site hypersensitivity.
Single or repeated injections of 99mTc-albumin macroaggregates may be associated with hypersensitive-type reactions including very rare life-threatening anaphylaxis, with chest pain, rigor and collapse. Local allergic reactions have been seen at the injection site.
Exposure to ionising radiation is linked with cancer induction and a potential for development of hereditary defects.
As the effective dose is 2.0 mSv when the maximal recommended activity of 185 MBq is administered these adverse reactions are expected to occur with a low probability.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via Yellow Card Scheme. Website: www.mhra.gov.uk/yellowcard.
4.9 Overdose
The number of MAA particles per adult patient must not exceed 1.5 x 106.
In the event of the administration of a radiation overdose with 99mTc-MAA, the absorbed dose to the patient should be reduced where possible by increasing the elimination of the radionuclide from the body by forced diuresis and frequent bladder voiding.
5. PHARMACOLOGICAL PROPERTIES
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Diagnostic radiopharmaceuticals, ATC code: V09EB01 Pharmacodynamic effects
At the chemical concentrations used for diagnostic examinations, 99mTc-MAA does not appear to have any pharmacodynamic activity.
5.2 Pharmacokinetic properties
Distribution
Following injection into a superficial vein of the systemic venous circulation, the macroaggregates are carried at the speed of this circulation to the first capillary filter, i.e. the capillary tree of the pulmonary artery system.
Organ uptake
The albumin macroaggregate particles do not penetrate the lung parenchyma (interstitial or alveolar) but remain in a temporary occlusive position in the lumen of the capillary. When pulmonary flow distribution is normal, the compound distributes over the entire pulmonary area following physiologic gradients; when district flow is altered the areas of reduced flow are reached by a proportionally smaller amount of particles. The technetium labelled macroaggregates remain in the lungs for variable periods of time, depending of the structure, size and number of particles.
Half-life
The disappearance of activity from the particles in the lungs is governed by a exponential law: the larger aggregate have a longer biological half-life, whereas particles between 5 and 90 pm in diameter have a half-life ranging from 2 to 8 hours.
The decrease in pulmonary concentration is caused by the mechanical break-down of the particles occluding the capillaries, stemming from the systo-diastolic pressure pulsations within the capillary itself.
Elimination
The products of macroaggregate break-down, once recirculated as albumin microcolloid, are quickly removed by the macrophages of the reticuloendothelial system, i.e. essentially the liver and the spleen.
The microcolloid is metabolised with introduction of the radioactive label (99mTc) into the systemic circulation from which it is removed and excreted in urine.
5.3 Preclinical safety data
Correlation exists between the size of the MAA and their toxic effects.
The pathophysiologic mechanism responsible for toxicity is shown to be the increase of the pulmonary blood pressure.
Toxicological studies with dogs have demonstrated that with a single IV injection of 20 to 25 mg/kg of MAA having 10 to 50 pm in diameter the first pulmonary signs of toxicity (e.g. tachypnea) were observed.
A sharp increase of the pulmonary blood pressure is noticed when 20 mg of less than 80 pm sized MAA are injected, where no significant pressure changes are recorded with 40 mg of less than 35 pm MAA particles.
With suspension of MAA up to 150 pm diameter, no blood pressure changes appear below 10 mg/kg, while larger diameter suspensions (up to 300 pm) typical blood pressure changes in pulmonary artery appear when the dose exceeds 5 mg/kg.
The repeated-dose toxicity studies performed in dogs show no detectable variations in the general behaviour of the animals.
No evidence of pathological changes in the main organs has been detected.
Mutagenicity studies, toxicity to reproduction and development studies and long-term carcinogenicity studies have not been carried out. There is no evidence in the literature of teratogenic, mutagenic or carcinogenic effect of the unlabelled product.
This medicinal product is not intended for regular or continuous administration.
6. PHARMACEUTICAL PARTICULARS
6.1. List of excipients
Human albumin Stannous chloride dihydrate Sodium chloride Under nitrogen atmosphere
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Human albumin Stannous chloride dihydrate Sodium chloride
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products except those mentioned in section 12.
6.3 Shelf life 1 year.
After radiolabelling: store in a refrigerator (2 °C - 8 °C). and use within 8 hours.
6.4 Special precautions for storage
Store the kit in a refrigerator (2 °C - 8 °C).
For storage conditions after radiolabelling of the medicinal product, see section 6.3.
Storage of radiopharmaceuticals should be in accordance with national regulation on radioactive materials.
6.5 Nature and contents of container
15 ml, colourless, European Pharmacopoeia type I, drawn glass vials, closed with rubber stoppers and aluminium capsules.
Pack size: 5 multidose vials.
6.6 Special precautions for disposal
General warning
Radiopharmaceuticals should be received, used and administered only by authorised persons in designated clinical settings. Their receipt, storage, use, transfer and disposal are subject to the regulations and/or appropriate licences of the competent official organisation.
Radiopharmaceuticals should be prepared in a manner which satisfies both radiation safety and pharmaceutical quality requirements. Appropriate aseptic precautions should be taken.
Contents of the vials are intended only for use in the preparation of 99mTc-MAA and are not to be administered directly to the patient without first undergoing the preparative procedure.
For instructions on extemporaneous preparation of the medicinal product before administration, see sections 12.
If at any time in the preparation of this product the integrity of this vial is compromised it should not be used.
Administration procedures should be carried out in a way to minimise risk of contamination of the medicinal product and irradiation of the operators. Adequate shielding is mandatory.
The content of the kit before extemporaneous preparation is not radioactive. However, after sodium pertechnetate (99mTc), Ph. Eur. is added, adequate shielding of the final preparation must be maintained.
The administration of radiopharmaceuticals creates risks for other persons from external radiation or contamination from spill of urine, vomiting etc. Radiation protection precautions in accordance with national regulations must therefore be taken.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
CIS bio international RN306, Saclay B.P. 32
91192 Gif-sur-Yvette Cedex FRANCE
8 MARKETING AUTHORISATION NUMBER(S)
PL 11876/0009
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 15 July 1996 Date of latest renewal: 11 June 2010
10 DATE OF REVISION OF THE TEXT
25/11/2015
11 DOSIMETRY
Technetium (99mTc) is produced by means of a (99Mo/99mTc) generator and decays with the emission of gamma radiation with a mean energy of 140 keV and a half-life of 6.02 hours to technetium (99Tc) which, in view of its long half-life of 2.13 x 105 years can be regarded as quasi stable.
The data listed below are from ICRP 80:
DOSE ABSORBED PER ACTIVITY ADMINISTERED (mGy / MBq)
|
Organ |
Adult |
15 year old |
10 year old |
5 year old |
1 year old |
|
Adrenals |
0.0068 |
0.0088 |
0.013 |
0.019E |
0.031 |
|
Bladder |
0.0087 |
0.011 |
0.014 |
0.016 |
0.030 |
|
Bone surfaces |
0.0051 |
0.0064 |
0.0091 |
0.014 |
0.026 |
|
Brain |
0.00092 |
0.0012 |
0.002 |
0.0032 |
0.0055 |
|
Breast |
0.005 |
0.0056 |
0.0099 |
0.014 |
0.021 |
|
Gall bladder |
0.0056 |
0.007 |
0.01 |
0.016 |
0.024 |
|
GI-tract | |||||
|
Stomach |
0.0037 |
0.0052 |
0.008 |
0.012 |
0.02 |
|
SI |
0.002 |
0.0026 |
0.0043 |
0.0068 |
0.012 |
|
Colon |
0.0019 |
0.0026 |
0.0043 |
0.0069 |
0.012 |
|
(ULI |
0.0022 |
0.0029 |
0.005 |
0.0083 |
0.014 |
|
(LLI |
0.0016 |
0.0021 |
0.0033 |
0.005 |
0.0095 |
|
Heart |
0.0096 |
0.013 |
0.018 |
0.025 |
0.038 |
|
Kidneys |
0.0037 |
0.0048 |
0.0072 |
0.011 |
0.018 |
|
Liver |
0.016 |
0.021 |
0.03 |
0.042 |
0.074 |
|
Lungs |
0.066 |
0.097 |
0.13 |
0.21 |
0.39 |
|
Muscles |
0.0028 |
0.0037 |
0.0052 |
0.0077 |
0.014 |
|
Oesophagus |
0.0061 |
0.0077 |
0.011 |
0.015 |
0.022 |
|
Ovaries |
0.0018 |
0.0023 |
0.0035 |
0.0054 |
0.01 |
|
Pancreas |
0.0056 |
0.0075 |
0.011 |
0.017 |
0.029 |
|
Red marrow |
0.0032 |
0.0038 |
0.0053 |
0.0072 |
0.012 |
|
Skin |
0.0015 |
0.0017 |
0.0027 |
0.0043 |
0.0078 |
|
Spleen |
0.0041 |
0.0055 |
0.0083 |
0.013 |
0.022 |
|
Testes |
0.0011 |
0.0014 |
0.0022 |
0.0033 |
0.0062 |
|
Thymus |
0.0061 |
0.0077 |
0.011 |
0.015 |
0.022 |
|
Thyroid |
0.0025 |
0.0033 |
0.0057 |
0.009 |
0.016 |
|
Uterus |
0.0022 |
0.0028 |
0.0042 |
0.006 |
0.011 |
|
Remaining organs |
0.0028 |
0.0036 |
0.005 |
0.0074 |
0.013 |
|
Effective dose (mSv/MBq) |
0.011 |
0.016 |
0.023 |
0.034 |
0.063 |
The effective dose resulting from the administration of an activity of 185 MBq of 99mTc-MAA for an adult weighing 70 kg is about 2.0 mSv.
For an administered activity of 185 MBq the typical radiation dose to the target organ, lungs, is 12.2 mGy and the typical radiation dose to the critical organs, adrenals, bladder, liver, pancreas, spleen, are 1.26, 1.61, 2.96, 1.04 and 0.76 mGy respectively.
12 INSTRUCTIONS FOR PREPARATION OF RADIOPHARMACEUTICALS
Usual precautions regarding sterility and radioprotection must be respected.
Withdrawals should be performed under aseptic conditions.
The vial must not be opened and must be kept inside the lead shielding. After disinfection of the stopper, the solution should be withdrawn via the stopper using a single dose syringe fitted with suitable protective shielding and a disposable sterile needle or using an authorised automated application system.
If the integrity of this vial is compromised, the product should not be used.
Method of preparation
Usual precautions regarding sterility and radioprotection should be respected.
Take a vial from the kit and put it in an appropriate lead shielding.
Using a hypodermic syringe, introduce through the rubber stopper 2.5 to 10 ml of sterile and pyrogen-free sodium pertechnetate [99mTc] injection, radioactivity varying as a function of the volume from 92.5 to maximum 3700 MBq.
Sodium pertechnetate [99mTc] injection should comply with European Pharmacopoeia specifications.
Do not use a breather needle as the contents is under nitrogen : after introduction of the volume of sodium pertechnetate [99mTc] injection, without removing the needle, withdraw an equivalent volume of nitrogen in order to avoid excess pressure in the vial.
Shake for about 2 minutes and wait for 15 minutes before use.
The vial should be shaked before each withdrawal in order to homogenise the suspension.
The syringe should be swirled immediately prior to injection to homogenise the injectate.
The homogeneousness of the suspension after preparation, pH, radioactivity and gamma spectrum should be checked before use.
The vial should never be opened and must be kept inside its lead shielding. The suspension should be removed aseptically through the stopper with a sterile lead protected syringe.
Determination of volume and activity of pertechnetate in relation with the number of MAA particles per dose.
In order to take into account the number of MAA particles per dose in the determination of volume and radioactivity of pertechnetate to prepare the radiopharmaceutical, charts have been performed and are described hereafter.
The proposed figures in the following tables are calculated from a mean value of 3 millions of MAA particles per vial.
- The first step allows to determine the volume of labelling of the vial as a function of the volume and the number of MAA particles to inject per dose. The used formula is as follows:
Number of MAA particles per vial x Volume to inject Voumeo a e mg - Number of MAA particles to inject per dose
The tables 1 and 2 show examples for volumes to inject of 0.5, 0.8 and 1 ml.
- The second step allows to know the radioactivity to add in the vial for the labelling as a function of the radioactivity to inject and the previously set parameters.The used formula is as follows:
. . Radioactivity to inject x Volume of labelling
Total radioactivity ofthevial = Volume to inject
The total radioactivity of the vial is calculated for radioactivities to inject of 37, 74, 111 and 148 MBq. See tables 3, 4, 5 and 6.
- The third step will describe the decrease calculation taking into account the time of labelling and the time of injection. The decay table of 99mTc is presented in table 7.
DETERMINATION OF THE LABELLING VOLUME FROM VOLUME AND NUMBER OF MAA PARTICLES TO INJECT AND CONSIDERING A VIAL CONTAINING 3 MILLIONS MAA PARTICLES

Injected volume (ml)
|
NUMBER OF MAA PARTICLES TO INJECT PER DOSE |
VOLUME TO INJECT (ml) | ||
|
0.5 |
0.8 |
1 | |
|
600 000 |
2.5 |
4 |
5 |
|
500 000 |
3 |
4.8 |
6 |
|
480 000 |
3.1 |
5 |
6.3 |
|
428 000 |
3.5 |
5.6 |
7 |
|
400 000 |
3.75 |
6 |
7.5 |
|
375 000 |
4 |
6.4 |
8 |
|
343 000 |
4.4 |
7 |
8.7 |
|
330 000 |
4.5 |
7.3 |
9 |
|
300 000 |
5 |
8 |
10 |
|
267 000 |
5.6 |
9 | |
|
250 000 |
6 |
9.6 | |
|
240 000 |
6.25 |
10 | |
|
215 000 |
7 | ||
|
188 000 |
8 | ||
|
167 000 |
9 | ||
|
150 000 |
10 | ||
Labelling volume (ml)
Number of MAA particles to inject / dose
DETERMINATION OF THE NUMBER OF INJECTED MAA PARTICLES AS A FUNCTION OF THE LABELLING VOLUME OF THE VIAL AND THE VOLUME TO INJECT AND CONSIDERING A VIAL CONTAINING 3 MILLIONS MAA
PARTICLES
|
VOLUME OF LABELLING (ml) |
VOLUME TO INJECT (ml) | ||
|
0.5 |
0.8 |
1 | |
|
3 |
500 000 | ||
|
4 |
375 000 |
600 000 | |
|
5 |
300 000 |
480 000 |
600 000 |
|
6 |
250 000 |
400 000 |
500 000 |
|
7 |
215 000 |
343 000 |
428 000 |
|
8 |
188 000 |
300 000 |
375 000 |
|
9 |
167 000 |
267 000 |
330 000 |
|
10 |
150 000 |
240 000 |
300 000 |
Labelling volume (mL) Injected volume (mL)
Number of MAA particles to inject/dose
DETERMINATION OF THE RADIOACTIVITY TO ADD TO THE VIAL AS A FUNCTION OF THE LABELLING VOLUME, THE VOLUME AND THE RADIOACTIVITY TO INJECT AND CONSIDERING A VIAL CONTAINING 3 MILLIONS MAA PARTICLES
|
37 MBq |
74 MBq |
111 MBq |
148 MBq |
|
0.5 |
0.1 |
1 | |
|
3 |
222 |
139 |
111 |
|
41 |
296 |
185 |
148 |
|
51 |
370 |
231 |
185 |
|
61 |
444 |
277 |
222 |
|
7 |
518 |
324 |
259 |
|
1 |
592 |
370 |
296 |
|
1 |
666 |
416 |
333 |
|
10 |
740 |
462 |
370 |
Table 3
|
0.5 |
0.1 |
1 |
|
444 | ||
|
592 |
370 | |
|
740 |
462 |
370 |
|
888 |
555 |
444 |
|
1036 |
647 |
518 |
|
1184 |
740 |
592 |
|
1332 |
832 |
666 |
|
1480 |
925 |
740 |
|
Table | ||
|
0.5 |
0.1 |
1 |
|
666 | ||
|
888 |
555 | |
|
1110 |
694 |
555 |
|
1332 |
832 |
666 |
|
1554 |
980 |
777 |
|
1776 |
1110 |
888 |
|
1998 |
1249 |
999 |
|
2220 |
1387 |
1110 |
Table 5
|
0.5 |
0.1 |
1 |
|
888 | ||
|
1184 |
740 | |
|
1480 |
925 |
740 |
|
1776 |
1110 |
888 |
|
2072 |
1295 |
1036 |
|
2368 |
1480 |
1184 |
|
2664 |
1665 |
1332 |
|
2960 |
1850 |
1480 |
Table 6
Injected activity (MBq)
Injected volume (ml)
Total activity (MBq)

Labelling volume (ml)
%
"Tc (HALF-LIFE : 6.02 hours) DECAY TABLE
H Min %
H Min % H Min % H Min % H Min
H Min
|
0 |
05 |
99.05 |
2 |
05 |
78.67 |
4 |
05 |
62 |
49 |
6 |
05 |
49 |
64 |
8 |
05 |
39.43 |
10 |
05 |
31 |
32 |
|
0 |
10 |
98.10 |
2 |
10 |
77.92 |
4 |
10 |
61 |
89 |
6 |
10 |
49 |
16 |
8 |
10 |
39.05 |
10 |
10 |
31 |
02 |
|
0 |
15 |
97.16 |
2 |
15 |
77.18 |
4 |
15 |
61 |
30 |
6 |
15 |
48 |
69 |
8 |
15 |
38.68 |
10 |
15 |
30 |
72 |
|
0 |
20 |
96.23 |
2 |
20 |
76.44 |
4 |
20 |
60 |
72 |
6 |
20 |
48 |
23 |
8 |
20 |
38.61 |
10 |
20 |
30 |
43 |
|
0 |
25 |
95.32 |
2 |
25 |
75.71 |
4 |
25 |
60 |
14 |
6 |
25 |
47 |
77 |
8 |
25 |
37.94 |
10 |
25 |
30 |
14 |
|
0 |
30 |
94.41 |
2 |
30 |
74.99 |
4 |
30 |
59 |
56 |
6 |
30 |
47 |
31 |
8 |
30 |
37.58 |
10 |
30 |
29 |
85 |
|
0 |
35 |
93.50 |
2 |
35 |
74.27 |
4 |
35 |
58 |
99 |
6 |
35 |
46 |
86 |
8 |
35 |
37.22 |
10 |
35 |
29 |
57 |
|
0 |
40 |
92.61 |
2 |
40 |
73.56 |
4 |
40 |
58 |
43 |
6 |
40 |
46 |
41 |
8 |
40 |
36.87 |
10 |
40 |
29 |
28 |
|
0 |
45 |
91.73 |
2 |
45 |
72.86 |
4 |
45 |
57 |
87 |
6 |
45 |
45 |
97 |
8 |
45 |
36.51 |
10 |
45 |
29 |
00 |
|
0 |
50 |
90.85 |
2 |
50 |
72.16 |
4 |
50 |
57 |
32 |
6 |
50 |
45 |
53 |
8 |
50 |
36.17 |
10 |
50 |
28 |
73 |
|
0 |
55 |
89.98 |
2 |
55 |
71.47 |
4 |
55 |
56 |
77 |
6 |
55 |
45 |
10 |
8 |
55 |
35.82 |
10 |
55 |
28 |
45 |
|
1 |
00 |
89.12 |
3 |
00 |
70.79 |
5 |
00 |
56 |
23 |
7 |
00 |
44 |
66 |
9 |
00 |
35.48 |
11 |
00 |
28 |
18 |
|
1 |
05 |
88.27 |
3 |
05 |
70.12 |
5 |
05 |
55 |
69 |
7 |
05 |
44 |
24 |
9 |
05 |
35.14 |
11 |
05 |
27 |
91 |
|
1 |
10 |
87.43 |
3 |
10 |
69.45 |
5 |
10 |
55 |
16 |
7 |
10 |
43 |
82 |
9 |
10 |
34.80 |
11 |
10 |
27 |
64 |
|
1 |
15 |
86.60 |
3 |
15 |
68.78 |
5 |
15 |
54 |
64 |
7 |
15 |
43 |
40 |
9 |
15 |
34.47 |
11 |
15 |
27 |
38 |
|
1 |
20 |
85.77 |
3 |
20 |
68.13 |
5 |
20 |
54 |
11 |
7 |
20 |
42 |
98 |
9 |
20 |
34.14 |
11 |
20 |
27 |
12 |
|
1 |
25 |
84.95 |
3 |
25 |
67.48 |
5 |
25 |
53 |
60 |
7 |
25 |
42 |
57 |
9 |
25 |
33.82 |
11 |
25 |
26 |
86 |
|
1 |
30 |
84.14 |
3 |
30 |
66.83 |
5 |
30 |
53 |
09 |
7 |
30 |
42 |
17 |
9 |
30 |
33.49 |
11 |
30 |
26 |
60 |
|
1 |
35 |
83.33 |
3 |
35 |
66.19 |
5 |
35 |
52 |
58 |
7 |
35 |
41 |
76 |
9 |
35 |
33.17 |
11 |
35 |
26 |
35 |
|
1 |
40 |
82.54 |
3 |
40 |
65.56 |
5 |
40 |
52 |
08 |
7 |
40 |
41 |
36 |
9 |
40 |
32.86 |
11 |
40 |
26 |
10 |
|
1 |
45 |
81.75 |
3 |
45 |
64.94 |
5 |
45 |
51 |
58 |
7 |
45 |
40 |
97 |
9 |
45 |
32.54 |
11 |
45 |
25 |
85 |
|
1 |
50 |
80.97 |
3 |
50 |
64.32 |
5 |
50 |
51 |
09 |
7 |
50 |
40 |
58 |
9 |
50 |
32.23 |
11 |
50 |
25 |
60 |
|
1 |
55 |
80.20 |
3 |
55 |
63.70 |
5 |
55 |
50 |
60 |
7 |
55 |
40 |
19 |
9 |
55 |
31.92 |
11 |
55 |
25 |
36 |
|
2 |
00 |
79.43 |
4 |
00 |
63.09 |
6 |
00 |
50 |
12 |
8 |
00 |
39 |
81 |
10 |
00 |
31.62 |
12 |
00 |
25 |
12 |
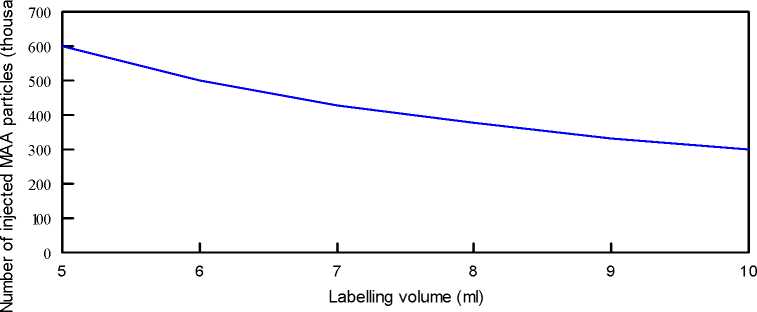
EXAMPLE FOR AN INJECTED VOLUME OF 1 ml
The following table and curve allow to determine the number of MAA particles injected when volumes of labelling are 5 to 10 ml and when the volume to inject is 1 ml.
The proposed figures in the following tables are calculated from a mean value of 3 millions of MAA particles per vial.
The formula used is:
Number of injected MAA particles =
T otal numb er of MAA particles x Injectedv olume Labelling volume
"O
c
|
Volume of labelling (ml) |
Number of injected MAA particles |
|
5 |
600 000 |
|
6 |
500 000 |
|
7 |
428 600 |
|
8 |
375 000 |
|
9 |
333 300 |
|
10 |
300 000 |
The following table and graph allow deducing the total radioactivity to add to the vial when the radioactivities to inject are 74, 111 or 148 MBq with a injected volume of 1 ml and considering a vial containing 3 millions particles.
|
Volume of |
Total radioactivity per vial (MBq) with a radioactivity to inject of | ||
|
labelling (ml) |
74 MBq |
111 MBq |
148 MBq |
|
5 |
370 |
555 |
740 |
|
6 |
444 |
666 |
888 |
|
7 |
518 |
777 |
1036 |
|
8 |
592 |
888 |
1184 |
|
9 |
666 |
999 |
1332 |
|
10 |
740 |
1110 |
1480 |
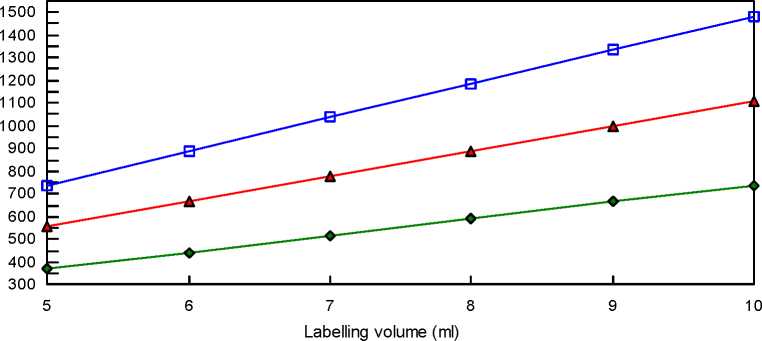
O Injected radioactivity = 74 MBq (2 mCi) a Injected radioactivity = 111 MBq (3 mCi) -B- Injected radioactivity = 148 MBq (4 mCi)
Quality control
The quality of labelling (radiochemical purity) could be checked according to the following procedure
Method
Non-filterable radioactivity. Materials and methods
1. Polycarbonate membrane filter 13 mm to 25 mm in diameter, 10 pm thick and with circular pores 3 pm in diameter.
2. 0.9 % sodium chloride solution.
3. Miscellaneous : syringes, needles, 15 ml glass vials, appropriate counting assembly. Procedure
1. Fit the membrane into a suitable holder.
2. Place 1 ml of the injection on the membrane, filter and collect in a vial (A).
3. Rinse the membrane with 2 ml of 0.9% sodium chloride solution and collect in the vial (A).
4. Measure the radioactivity of the filter (X) and the radioactivity of the vial A (Y), using an appropriate detection apparatus.
5. Calculations :
Calculate the percentage of technetium [99mTc] human albumin macroaggregates as follows :
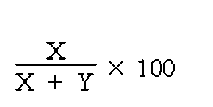
The radioactivity remaining on the membrane should be not less than 90 % of the total radioactivity of the injection.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Detailed information on this medicinal product is available on the website of the MHRA.