Rhinocort Hay-Fever 64 Micrograms Nasal Spray
Package leaflet: Information for the user
Rhinocort® Hay Fever, 64 micrograms, Nasal Spray
Budesonide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
This medicine is available without prescription. Always use this medicine exactly as described in this leaflet or as your pharmacist has told you.
• Keep this leaflet. You may need to read it again.
• Ask your pharmacist if you need more information or advice.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.See section 4.
• You must talk to a doctor if you do not feel better or if you feel worse after 14 days.
What is in this leaflet
1. What Rhinocort Hay Fever is and what it is used for
2. What you need to know before you use Rhinocort Hay Fever
3. How to use Rhinocort Hay Fever
4. Possible side effects
5. How to store Rhinocort Hay Fever
6. Contents of the pack and other information
1. What Rhinocort Hay Fever is and what it is used for
This nasal spray contains a medicine called budesonide. This belongs to a group of medicines called corticosteroids.
• Corticosteroids help to dampen down and prevent inflammation.
• This nasal spray is used to treat inflammation in the nose that can occur as a result of an allergy to things such as pollen. The inflammation causes the nose to be blocked and results in sneezing, these are typical symptoms of hay fever.
• Regular use of this nasal spray over several days can reduce inflammation in the nose and help prevent these symptoms.
You must talk to a doctor if you do not feel better or if you feel worse after 14 days.
2. What you need to know you use Rhinocort Hay Fever
Do not use Rhinocort Hay Fever:
• If you are allergic to budesonide or any of the other ingredients of this medicine (listed in section 6).
• If you are under 18 years of age.
Do not use this nasal spray if any of the above apply to you. If you are not sure, talk to your doctor or pharmacist before using it.
Warnings and precautions
Talk to your doctor or pharmacist before using Rhinocort Hay Fever if:
• You have an infection of your nose or sinuses.
• You have ulcers in your nose.
• You have recently had surgery to your nose.
• You have TB (tuberculosis).
• You have a chest infection.
• You have reduced liver function.
If any of the above apply to you, or if you are not sure, talk to your doctor or pharmacist before using this nasal spray.
Other medicines and Rhinocort Hay Fever
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This includes medicines that you buy without a prescription and herbal medicines.
In particular, talk to your doctor or pharmacist if:
• You are taking medicines to treat fungal infections (such as itraconazole or ketoconazole).
• You have been taking steroid tablets. Your doctor or pharmacist may ask you to take fewer of them after starting to use this nasal spray.
• You are taking oestrogens and contraceptive steroids.
Pregnancy, breast-feeding and fertility
• If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
• If you get pregnant while using this nasal spray, talk to your doctor or pharmacist straight away.
Driving and using machines
Rhinocort Hayfever is not likely to affect you being able to drive or use any tools or machines.
3. How to use Rhinocort Hay Fever
Always use this medicine exactly as described in this leaflet or as your doctor or pharmacist have told you. Check with your doctor or pharmacist if you are not sure.
How much Rhinocort Hay Fever to use
Do not use more than two sprays into each nostril in a day.
Using the nasal spray once a day:
• The recommended dose is two sprays into each nostril in the morning.
• If your symptoms improve, reduce this to one spray in each nostril in the morning.
Using the nasal spray twice a day:
• If you prefer, you can use one spray into each nostril every morning and evening. When to start using the nasal spray
Try to start using this nasal spray before you come into contact with whatever causes the allergy. For example, if it is a particular type of pollen that causes your hay fever, use this nasal spray just before and during the time when you are exposed to it.
How long to use the nasal spray for
• Use this nasal spray regularly. Keep using it until you are no longer exposed to whatever is causing your allergy.
• The full effect of treatments like this nasal spray are not achieved until after a few days of treatment. You may not notice any effects for the first few days.
• If there is no improvement in your symptoms after 14 days, talk to your doctor or pharmacist.
• Do not use this nasal spray continuously for longer than 3 months without talking to your doctor.
Use in children
This nasal spray should not be used in children and adolescents under 18 years.
Instructions for use
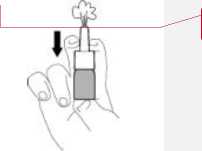
1. Priming the nozzle
Commented [A1]: Element Item Number: 144260.1209. Please place at end of line.
Before using this nasal spray for the first time, you must prime the nozzle (that is, fill it with the medicine). To do this:
• Shake the bottle and remove the protective cap.
• Hold the bottle upright as shown in the picture.
• Pump the nozzle up and down several times (5 to 10 times), spraying into the air until you see an even mist.
The priming effect remains for about 24 hours. If you leave a gap of more than a day before the next dose, you will need to prime the nozzle again. This time spray it into the air just once.
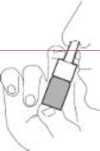
2. Using Rhinocort Hay Fever
• Blow your nose. Shake the bottle. Remove the protective cap.
Commented [A2]:
place at end of line.
Element Item Number: 144261.1209. Please
• Hold the bottle as shown in the picture.
• Put the tip of the nozzle into your nostril,
as shown. Spray once (or more if the doctor or pharmacist has told you to). Use the spray in the other nostril in the same way. Note, you do not need to breathe in at the same time as you spray.
• Wipe the nozzle with a clean tissue and replace
the protective cap. Store the bottle in an upright position.
3. Cleaning the nozzle
It is best to clean the plastic nozzle of this nasal spray regularly. Also clean it if you notice that the spray is not coming out as it should. If this happens, first check the nozzle is primed with medicine (see “Priming the nozzle” above). If after priming the nozzle again the pump is still not working, clean the nozzle as follows:
• Remove the plastic nozzle with a clean tissue and wash it in warm (not hot) water.
• Rinse the nozzle thoroughly, dry it and then replace it on the top of the bottle. Never try to unblock the nozzle by using a pin or other sharp object.
• Prime the nozzle again (by filling it with medicine) before use.
Additional information about using Rhinocort Hay Fever
• If you are about to have an operation or during times of stress, please tell your doctor that you use this nasal spray. Your doctor may ask you to take steroid tablets as well,
particularly if you have been taking a high dose of this nasal spray, or a similar medicine, for a long time.
• Eye problems: If you have an allergy, which is also causing eye problems, you may need to take another medicine as well.
If you use more Rhinocort Hay Fever than you should
It is important that you take your dose as stated on the pharmacist’s label or as advised by your doctor. You should use only as much as your doctor recommends; using more or less may make your symptoms worse.
If you take too many doses of this nasal spray, talk to your doctor or pharmacist straight away.
If you forget to use Rhinocort Hay Fever
If you forget to take a dose, take it as soon as you remember. However, if it is almost time to take the next dose, wait until then. Do not take a double dose to make up for a forgotten dose.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If any of the following happen to you, stop using this nasal spray and talk to your doctor straight away:
• Skin rash, itching, lumpy rash (hives), blistering of the skin. Swelling of your face, lips, tongue or throat (this may make it difficult to swallow). These are uncommon, affecting less than 1 in 100 people.
• Severe allergic reaction. This is rare, affecting less than 1 in 1000 people.
• In addition to the above, sudden wheezing and/or feeling faint may occur. It is not known how often these occur; however they are very unlikely.
If any of the above occurs, you may be having an allergic reaction.
Other possible side effects:
Common (may affect up to 1 in 10 people)
• Occasional sneezing, dry nose, or stinging in your nose. These can happen straight after using this nasal spray.
• Slight bleeding from the nose.
Uncommon (may affect up to 1 in 100 people)
• Muscle cramps
Rare (may affect up to 1 in 1000 people)
• Slowing in the rate of growth of children and adolescents. Corticosteroids can affect the normal production of steroid hormones in your body, particularly if you use high doses for a long time. This is much less likely to happen with inhaled corticosteroids than with corticosteroid tablets.
• Perforation of the membrane separating the nostrils (the nasal septum).
• Nasal ulcer.
• Loss of voice.
• Bruising of the skin.
Very rare (may affect up to 1 in 10,000 people)
• Sore patches on the inside of the nose.
Not known (frequency cannot be estimated from available data)
• Glaucoma (increased pressure in the eye).
• Cataract
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can report side effects directly via the Yellow Card Scheme. Website: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Rhinocort Hay Fever
• Keep this medicine out of the sight and reach of children.
• Do not use this medicine after the expiry date which is stated on the carton. The expiry date refers to the last day of the month.
• Do not store above 30°C.
• Do not freeze or store in the fridge.
• Store the bottle in an upright position.
• Use within 2 months of opening the bottle. You may find it helpful to write on this leaflet when you first opened the bottle:
Date bottle opened...............................................
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Rhinocort Hay Fever contains
• The active ingredient is budesonide. This nasal spray contains 64 micrograms of budesonide in each spray.
• The other ingredients are disodium edetate, potassium sorbate (E202), glucose (anhydrous), microcrystalline cellulose (E460), carboxymethylcellulose sodium (E466), polysorbate 80 (E433), hydrochloric acid and purified water.
• Each bottle of this nasal spray contains 120 sprays.
•
What Rhinocort Hay Fever looks like and contents of the pack
This nasal spray is a metered pump nasal spray. Rhinocort Hay Fever is available in a 10 ml amber/brown glass bottle. Each bottle is fitted with a spray pump and contains 120 sprays.
Marketing Authorisation Holder and Manufacturer
The Marketing Authorisation Holder for Rhinocort Hay Fever marketed in the UK is AstraZeneca UK Limited, 600 Capability Green, Luton, LU1 3LU.
Rhinocort Hay Fever is manufactured by AstraZeneca UK Limited, Silk Road Business Park, Macclesfield, Cheshire, SK10 2NA, UK.
This leaflet was last revised in August 2016
©AstraZeneca 2016
Rhinocort is a trademark of the AstraZeneca group of companies.
RSP 16 0036a
To listen to or request a copy of this leaflet in Braille, large print or audio please call, free of charge:
0800 198 5000 (UK only)
Please be ready to give the following information:
Product name Rhinocort Hay Fever 64 micrograms
Reference number 17901/0254
This is a service provided by the Royal National Institute of Blind People.
UK PIL Rhinocort Hayfever 64 RSP 16 0036a (based on RSP 15 0029) 18/08/2016 KV Page 6 of6