Roxina 0.02 Mg/3 Mg Film-Coated Tablets
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Roxina 0.02 mg/3 mg film-coated tablets
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each film-coated tablet contains 0.02 mg ethinylestradiol and 3 mg drospirenone. Excipients with known effect:
Each film-coated tablet contains 46.106 mg lactose (as 48.53 mg of lactose monohydrate) and 0.070 mg of soya lecithin.
For the full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Film-coated tablets
The active tablet is white or almost white, round, biconvex film-coated tablet, diameter about 6 mm. Engraving on one side: “G73”, the other side is without engraving.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Oral contraception
The decision to prescribe Roxina should take into consideration the individual woman’s current risk factors, particularly those for venous thromboembolism (VTE), and how the risk of VTE with Roxina compares with other combined hormonal contraceptives (CHCs) (see sections 4.3 and 4.4).
4.2 Posology and method of administration
Before using Roxina for the first time, patients should be advised how to use this product during the different phases.
Method of administration: oral use
Posology
How to take Roxina
The tablets must be taken every day at about the same time, if necessary with a small amount of liquid.
Tablets must always be taken continuously for a minimum of 24 days. Roxina can be taken continuously for up to 120 days, after which a 4-day tablet-free interval must be started.
The tablet taking consists of two phases:
1. A mandatory phase (day 1-24):
When starting Roxina, tablets must be taken continuously for a minimum of 24 days, after which the woman can either:
- take a 4-days tablet-free interval
- or continue to take the tablets for up to 120 days (see flexible phase below).
2. A flexible phase (day 25-120):
During the days 25-120, the tablets can be taken continuously up to a maximum of 120 days (when all the blisters included in this package will be finished). Within this period, the woman may decide herself to take the 4-day tablet-free interval (to schedule the withdrawal bleeding). This 4-day-tablet-free-interval should only be started if tablet taking has been continuous for 24 days.
In the event of continued bleeding (three consecutive days) during the flexible phase (days 25-120), it is advisable to take the 4-day table-free interval which will induce a withdrawal bleeding. This will reduce the total number of days with bleeding.
Tablet-free interval
A tablet-free interval should never be longer than 4 days and it should only be started if tablet taking has been continuous for 24 days.
During the 4-day tablet-free interval bleeding usually occurs and may not have finished before the woman starts the next tablet intake cycle.
After each 4-day tablet-free interval, a new intake cycle starts of a minimum of 24 days to a maximum of 120 days. After the mandatory phase of 24 days of continuous tablet taking, the woman again may choose to have the tablet-free 4 day interval or not, until day 120 of the flexible phase has been reached.
No 4-day tablet-free interval in the flexible phase
A 4-day tablet-free interval should never be later than after 120 days of continuous tablet intake.
Intake in the mandatory phase
The woman must start a new blister, which contains 24 tablets, for the mandatory phase and after a tablet free interval, in order to guide the woman to correctly follow the product administration.
Intake in the flexible phase
If the woman wishes to use the partial blister pack she must use the partial blister pack (if available) at the beginning of the flexible phase immediately following the mandatory phase started after her last 4-day tablet-free interval.
The prescription of the next blister pack should be issued in time i.e. prior to use of the last blister in the pack to ensure that the woman does not run out of tablets.
How to start Roxina - No preceding hormonal contraceptive use (in the past month)
Tablet-taking has to start on day 1 of the woman’s natural cycle (i.e. the first day of her menstrual
bleeding). Starting on days 2-5 is allowed, but during the first cycle a barrier method is recommended in addition for the first 7 days of tablet-taking.
- Changing from a combined hormonal contraceptive (combined oral
contraceptive (COC), vaginal ring or transdermal patch)
The woman should start with Roxina preferably on the day after the last active tablet (the last tablet containing the active substances) of her previous COC, but at the latest on the day following the usual tablet-free or hormone-free tablet interval of her previous COC. In case a vaginal ring or transdermal patch has been used the woman should start using Roxina preferably after removal, but at the latest when the next application would have been due.
Changing from a progestogen-only-method (progestogen-only pill, injection, implant) or from a progestogen-releasing intrauterine system (IUS)
The woman may switch any day from the progestogen-only pill (from an implant or the IUS on the day of its removal, from an injectable when the next injection would be due) but should in all of these cases be advised to additionally use a barrier method for the first 7 days of tablet-taking.
- Following first-trimester abortion
The woman may start immediately. When doing so, she need not take additional contraceptive measures.
- Following delivery or second-trimester abortion
Women should be advised to start at day 21 to 28 after delivery or second-trimester abortion. When starting later, the woman should be advised to additionally use a barrier method for the first 7 days of tablet-taking. However, if intercourse has already occurred, pregnancy should be excluded before the actual start of COC use or the woman has to wait for her first menstrual period.
For breastfeeding women see section 4.6.
Management of missed tablets
If the user is less than 24 hours late in taking any tablet, contraceptive protection is not reduced. The woman should take the tablet as soon as she remembers and should take further tablets at the usual time.
If she is more than 24 hours late in taking any tablet, contraceptive protection may be reduced. The management of missed tablets can be guided by the following two basic rules:
1. the recommended tablet-free interval is 4 days, tablet-taking must never be discontinued for longer than 7 days
2. 7 days of uninterrupted tablet-taking are required to attain adequate suppression of the hypothalamic-pituitary-ovarian-axis.
Accordingly the following advice can be given in daily practice:
Day 1-7
The user should take the last missed tablet as soon as she remembers, even if this means taking two tablets at the same time. She then continues to take tablets at her usual time. In addition, a barrier method such as a condom should be used for the next 7 days. If intercourse took place in the preceding 7 days, the possibility of a pregnancy should be considered. The more tablets are missed and the closer they are to the tablet-free interval, the higher the risk of a pregnancy.
Day 8-24
The user should take the last missed tablet as soon as she remembers, even if this means taking two tablets at the same time. She then continues to take tablets at her usual time. Provided that the woman has taken her tablets correctly in the 7 days preceding the first missed tablet, there is no need to use extra contraceptive precautions. However, if this is not the case or if she has missed more than 1 tablet, the woman should be advised to use extra precautions until she has taken tablets continuously without interruption for at least 7 days.
Day 25-120
The risk of reduced reliability may be imminent because of the possibility of a forthcoming hormone-free interval. However, by adjusting the tablet-intake schedule, reduced contraceptive protection can still be prevented. By adhering to either of the following two options, there is therefore no need to use extra contraceptive precautions, provided that in the 7 days preceding the first missed tablet the woman has taken all tablets correctly. If this is not the case, the woman should be advised to follow the first of these two options and to use extra precautions for the next 7 days as well.
1. The user should take the last missed tablet as soon as she remembers, even if this means taking two tablets at the same time. She then continues to take tablets at her usual time until she has taken at least 7 tablets in a row without interruption.
2. The woman may also decide to have a tablet-free interval of 4 days, including the days she missed tablets, in order to induce the withdrawal bleeding and subsequently start a new intake cycle of Roxina.
If the woman missed tablets and subsequently has no withdrawal bleed in the following tablet-free interval, the possibility of a pregnancy should be considered.
Advice in case of gastro-intestinal disturbances
In case of severe gastro-intestinal disturbances (e.g., vomiting or diarrhoea), absorption may not be complete and additional contraceptive measures should be taken.
If vomiting occurs within 3-4 hours after taking a tablet the advice concerning missed tablets, as given in section 4.2, is applicable. If the woman does not want to change her tablet-taking schedule, she has to take the extra tablet(s) from another blister pack.
Additional information on special populations
Pediatric population
Roxina is only indicated after menarche.
Elderly
Not applicable. Roxina is not indicated after menopause.
Hepatic impairment
Roxina is contraindicated in women with severe hepatic diseases. See also sections
4.3 and 5.2.
Renal impairment
Roxina is contraindicated in women with severe renal insufficiency or acute renal failure. See also sections 4.3 and 5.2.
4.3 Contraindications
Combined hormonal contraceptives (CHCs) should not be used in of the following conditions. Should any of the conditions appear for the first time during CHC use, the product should be stopped immediately.
- Presence or risk of venous thromboembolism (VTE)
- Venous thromboembolism - current VTE (on anticoagulants) or history of (e.g. deep venous thrombosis [DVT] or pulmonary embolism [PE]).
- Known hereditary or acquired predisposition for venous thromboembolism, such as APC-resistance, (including Factor V Leiden), antithrombin-III-deficiency, protein C deficiency, protein S deficiency
- Major surgery with prolonged immobilisation (see section 4.4)
- A high risk of venous thromboembolism due to the presence of multiple risk factors (see section 4.4)
- Presence or risk of arterial thromboembolism (ATE)
- Arterial thromboembolism - current arterial thromboembolism, history of arterial thromboembolism (e.g. myocardial infarction) or prodromal condition (e.g. angina pectoris).
- Cerebrovascular disease - current stroke, history of stroke or prodromal condition (e.g. transient ischaemic attack, TIA)
- Known hereditary or acquired predisposition for arterial thromboembolism, such as hyperhomocysteinaemia and antiphospholipid-antibodies (anticardiolipin-antibodies, lupus anticoagulant).
- History of migraine with focal neurological symptoms.
- A high risk of arterial thromboembolism due to multiple risk factors (see section 4.4) or to the presence of one serious risk factor such as:
- diabetes mellitus with vascular symptoms
- severe hypertension
- severe dyslipoproteinaemia
- Presence or history of severe hepatic disease as long as liver function values have not returned to normal
- Severe renal insufficiency or acute renal failure
- Presence or history of liver tumours (benign or malignant)
- Known or suspected sex-steroid influenced malignancies (e.g. of the genital organs or the breasts)
- Undiagnosed vaginal bleeding
- Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
- Hypersensitivity to peanut or soya.
4.4 Special warnings and precautions for use Warnings
If any of the conditions or risk factors mentioned below is present, the suitability of Roxina should be discussed with the woman.
In the event of aggravation, or first appearance of any of these conditions or risk factors, the woman should be advised to contact her doctor to determine whether the use of Roxina should be discontinued.
In case of suspected or confirmed VTE or ATE, CHC use should be discontinued. In case anticoagulant therapy is started, adequate alternative contraception should be initiated because of the teratogenicity of anticoagulant therapy (coumarins).
• Circulatory Disorders
Risk of venous thromboembolism (VTE)
The use of any combined hormonal contraceptive (CHC) increases the risk of venous thromboembolism (VTE) compared with no use. Products that contain levonorgestrel, norgestimate or norethisterone are associated with the lowest risk of VTE. Other products such as Roxina may have up to twice this level of risk. The decision to use any product other than one with the lowest VTE risk should be taken only after a discussion with the woman to ensure she understands the risk of VTE with Roxina, how her current risk factors influence this risk, and that her VTE risk is highest in the first ever year of use. There is also some evidence that the risk is increased when a CHC is re-started after a break in use of 4 weeks or more.
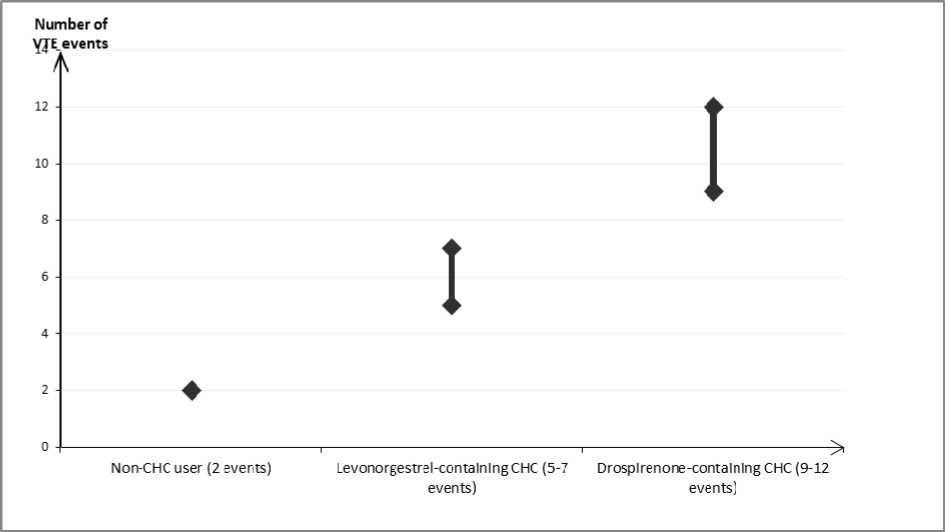
In women who do not use a CHC and are not pregnant about 2 out of 10,000 will develop a VTE over the period of one year. However, in any individual woman the risk may be far higher, depending on her underlying risk factors (see below).
It is estimated1 that out of 10,000 women who use a CHC containing drospirenone, between 9 and 12 women will develop a VTE in one year; this compares with about 62 in women who use a levonorgestrel-containing CHC.
In both cases, the number of VTEs per year is fewer than the number expected during pregnancy or in the postpartum period.
VTE may be fatal in 1-2% of cases.
Number of VTE events per 10,000 women in one year
Extremely rarely, thrombosis has been reported to occur in CHC users in other blood vessels, e.g. hepatic, mesenteric, renal or retinal veins and arteries.
Risk factors for VTE
The risk for venous thromboembolic complications in CHC users may increase substantially in a woman with additional risk factors, particularly if there are multiple risk factors (see table).
Roxina is contraindicated if a woman has multiple risk factors that put her at high risk of venous thrombosis (see section 4.3). If a woman has more than one risk factor, it is possible that the increase in risk is greater than the sum of the individual factors - in this case her total risk of VTE should be considered. If the balance of benefits and risks is considered to be negative a CHC should not be prescribed (see section 4.3).
|
Table: Risk factors for VTE | |
|
Risk factor |
Comment |
|
Obesity (body mass index over 30 kg/m2) |
Risk increases substantially as BMI rises. Particularly important to consider if other risk factors also present. |
|
Prolonged immobilisation, major surgery, any surgery to the legs or pelvis, neurosurgery, or major trauma Note: temporary immobilisation including air travel >4 hours can also be a risk factor for VTE, particularly in women with other risk factors |
In these situations it is advisable to discontinue use of the pill (in the case of elective surgery at least four weeks in advance) and not resume until two weeks after complete remobilisation. Another method of contraception should be used to avoid unintentional pregnancy. Antithrombotic treatment should be considered if Roxina has not been discontinued in advance. |
|
Positive family history (venous thromboembolism ever in a sibling or parent especially at a relatively early age e.g. before 50). |
If a hereditary predisposition is suspected, the woman should be referred to a specialist for advice before deciding about any CHC use. |
|
Other medical conditions associated with VTE |
Cancer, systemic lupus erythematosus, haemolytic uraemic syndrome, chronic inflammatory bowel disease (Crohn’s disease or ulcerative colitis) and sickle cell disease |
|
Increasing age |
Particularly above 35 years |
There is no consensus about the possible role of varicose veins and superficial thrombophlebitis in the onset or progression of venous thrombosis.
The increased risk of thromboembolism in pregnancy, and particularly the 6 week period of the puerperium, must be considered (for information on “Pregnancy and lactation” see section 4.6).
Symptoms of VTE (deep vein thrombosis and pulmonary embolism)
In the event of symptoms women should be advised to seek urgent medical attention and to inform the healthcare professional that she is taking a CHC.
Symptoms of deep vein thrombosis (DVT) can include:
- unilateral swelling of the leg and/or foot or along a vein in the leg;
- pain or tenderness in the leg which may be felt only when standing or walking;
- increased warmth in the affected leg; red or discoloured skin on the leg.
Symptoms of pulmonary embolism (PE) can include:
- sudden onset of unexplained shortness of breath or rapid breathing;
- sudden coughing which may be associated with haemoptysis;
- sharp chest pain;
- severe light headedness or dizziness;
- rapid or irregular heartbeat.
Some of these symptoms (e.g. “shortness of breath”, “coughing”) are non-specific and might be misinterpreted as more common or less severe events (e.g. respiratory tract infections).
Other signs of vascular occlusion can include: sudden pain, swelling and slight blue discoloration of an extremity.
If the occlusion occurs in the eye symptoms can range from painless blurring of vision which can progress to loss of vision. Sometimes loss of vision can occur almost immediately.
Risk of arterial thromboembolism (ATE)
Epidemiological studies have associated the use of CHCs with an increased risk for arterial thromboembolism (myocardial infarction) or for cerebrovascular accident (e.g. transient ischaemic attack, stroke). Arterial thromboembolic events may be fatal.
Risk factors for ATE
The risk of arterial thromboembolic complications or of a cerebrovascular accident in CHC users increases in women with risk factors (see table). Roxina is contraindicated if a woman has one serious or multiple risk factors for ATE that puts her at high risk of arterial thrombosis (see section 4.3). If a woman has more than one risk factor, it is possible that the increase in risk is greater than the sum of the individual factors - in this case her total risk should be considered. If the balance of benefits and risks is considered to be negative a CHC should not be prescribed (see section 4.3).
Table: Risk factors for ATE
|
Risk factor |
Comment |
|
Increasing age |
Particularly above 35 years |
|
Smoking |
Women should be advised not to smoke if they wish to use a CHC. Women over 35 who continue to smoke should be strongly advised to use a different method of contraception. |
|
Hypertension | |
|
Obesity (body mass index over 30 kg/m2) |
Risk increases substantially as BMI increases. Particularly important in women with additional risk factors. |
|
Positive family history (arterial thromboembolism ever in a sibling or parent especially at relatively early age e.g. below 50). |
If a hereditary predisposition is suspected, the woman should be referred to a specialist for advice before deciding about any CHC use. |
|
Migraine |
An increase in frequency or severity of migraine during CHC use (which may be prodromal of a cerebrovascular event) may be a reason for immediate discontinuation. |
|
Other medical conditions associated with adverse vascular events |
Diabetes mellitus, hyperhomocysteinaemia, valvular heart disease and atrial fibrillation, dyslipoproteinaemia and systemic lupus erythematosus. |
Symptoms of ATE
In the event of symptoms women should be advised to seek urgent medical attention and to inform the healthcare professional that she is taking a CHC.
Symptoms of a cerebrovascular accident can include:
- sudden numbness or weakness of the face, arm or leg, especially on one side of the body;
- sudden trouble walking, dizziness, loss of balance or coordination;
- sudden confusion, trouble speaking or understanding;
- sudden trouble seeing in one or both eyes;
- sudden, severe or prolonged headache with no known cause;
- loss of consciousness or fainting with or without seizure.
Temporary symptoms suggest the event is a transient ischaemic attack (TIA).
Symptoms of myocardial infarction (MI) can include:
- pain, discomfort, pressure, heaviness, sensation of squeezing or fullness in the chest, arm, or below the breastbone;
- discomfort radiating to the back, jaw, throat, arm, stomach;
- feeling of being full, having indigestion or choking;
- sweating, nausea, vomiting or dizziness;
- extreme weakness, anxiety, or shortness of breath;
- rapid or irregular heartbeats.
• Tumours
An increased risk of cervical cancer in long-term users of COCs (> 5 years) has been reported in some epidemiological studies, but there continues to be controversy about the extent to which this finding is attributable to the confounding effects of sexual behaviour and other factors such as human papilloma virus (HPV).
A meta-analysis from 54 epidemiological studies reported that there is a slightly increased relative risk (RR = 1.24) of having breast cancer diagnosed in women who are currently using COCs. The excess risk gradually disappears during the course of the 10 years after cessation of COC use. Because breast cancer is rare in women under 40 years of age, the excess number of breast cancer diagnoses in current and recent COC users is small in relation to the overall risk of breast cancer. These studies do not provide evidence for causation. The observed pattern of increased risk may be due to an earlier diagnosis of breast cancer in COC users, the biological effects of COCs or a combination of both. The breast cancers diagnosed in ever-users tend to be less advanced clinically than the cancers diagnosed in never-users.
In rare cases, benign liver tumours, and even more rarely, malignant liver tumours have been reported in users of COCs. In isolated cases, these tumours have led to life-threatening intra-abdominal haemorrhages. A hepatic tumour should be considered in the differential diagnosis when severe upper abdominal pain, liver enlargement or signs of intra-abdominal haemorrhage occur in women taking COCs.
With the use of the higher-dosed COCs (50 ^g ethinylestradiol) the risk of endometrial and ovarian cancer is reduced. Whether this also applies to lower-dosed COCs remains to be confirmed.
• Other conditions
The progestin component in in Roxina is an aldosterone antagonist with potassium sparing properties. In most cases, no increase of potassium levels is to be expected. In a clinical study, however in some patients with mild or moderate renal impairment and concomitant use of potassium-sparing medicinal products serum potassium levels slightly, but not significantly, increased during drospirenone intake. Therefore, it is recommended to check serum potassium during the first treatment cycle in patients presenting with renal insufficiency and a pretreatment serum potassium in the upper reference range, and particularly during concomitant use of potassium sparing medicinal products. See also section 4.5.
Women with hypertriglyceridemia, or a family history thereof, may be at an increased risk of pancreatitis when using COCs.
Although small increases in blood pressure have been reported in many women taking COCs, clinically relevant increases are rare. Only in these rare cases an immediate discontinuation of COC use is justified. If, during the use of a COC in preexisting hypertension, constantly elevated blood pressure values or a significant increase in blood pressure do not respond adequately to antihypertensive treatment, the COC must be withdrawn. Where considered appropriate, COC use may be resumed if normotensive values can be achieved with antihypertensive therapy.
The following conditions have been reported to occur or deteriorate with both pregnancy and COC use, but the evidence of an association with COC use is inconclusive: jaundice and/or pruritus related to cholestasis; gallstones; porphyria; systemic lupus erythematosus; haemolytic uremic syndrome; Sydenham's chorea; herpes gestationis; otosclerosis-related hearing loss.
In women with hereditary angioedema exogenous estrogens may induce or exacerbate symptoms of angioedema.
Acute or chronic disturbances of liver function may necessitate the discontinuation of COC use until markers of liver function return to normal. Recurrence of cholestatic jaundice and/or cholestasis-related pruritus which previously occurred during pregnancy or during previous use of sex steroids necessitates the discontinuation of COCs.
Although COCs may have an effect on peripheral insulin resistance and glucose tolerance, there is no evidence for a need to alter the therapeutic regimen in diabetics using low-dose COCs (containing < 0.05 mg ethinylestradiol). However, diabetic women should be carefully observed, particularly in the early stage of COC use.
Worsening of endogenous depression, of epilepsy, of Crohn's disease and of ulcerative colitis has been reported during COC use.
Chloasma may occasionally occur, especially in women with a history of chloasma gravidarum. Women with a tendency to chloasma should avoid exposure to the sun or ultraviolet radiation whilst taking COCs.
Medical examination/consultation
Prior to the initiation or reinstitution of Roxina a complete medical history (including family history) should be taken and pregnancy must be ruled out. Blood pressure should be measured and a physical examination should be performed, guided by the contra-indications (see section 4.3) and warnings (see section 4.4). It is important to draw a woman’s attention to the information on venous and arterial thrombosis, including the risk of Roxina compared with other CHCs, the symptoms of VTE and ATE, the known risk factors and what to do in the event of a suspected thrombosis.
The woman should also be instructed to carefully read the user leaflet and to adhere to the advice given. The frequency and nature of examinations should be based on established practice guidelines and be adapted to the individual woman.
Women should be advised that hormonal contraceptives do not protect against HIV infections (AIDS) and other sexually transmitted diseases.
Reduced efficacy
The efficacy of COCs may be reduced in the event of e.g. missed active tablets (see section 4.2), gastro-intestinal disturbances during active tablet taking (see section 4.2) or concomitant medication (see section 4.5).
Reduced cycle control
The flexible regimen is designed to delay menstruation. In many women, delay of menstruation is limited by the occurrence of spotting or breakthrough bleeding. The onset of this type of bleeding is unscheduled and cannot be predicted. Only the hormonal withdrawal bleeding which follows the 4-day tablet-free interval can be scheduled. The flexible regimen of Roxina allows scheduling of the withdrawal bleeding during the flexible phase between day 25 and 120 of the intake cycle.
As with all COCs, irregular bleeding (spotting or breakthrough bleeding) may occur, even during the fixed phase of the intake cycle between days 1-24, especially during the first months of use. Therefore, the evaluation of any irregular bleeding is only meaningful after an adaptation interval of about three months.
If bleeding irregularities persist even after induction of withdrawal bleeding, i.e. if bleeding episodes after the 4-day break are markedly prolonged or increased in intensity above the usual, then non-hormonal causes should be considered and adequate diagnostic measures are indicated to exclude malignancy or pregnancy. These may include curettage.
In some women withdrawal bleeding may not occur during the tablet-free interval. If the COC has been taken according to the directions described in section 4.2, it is unlikely that the woman is pregnant.
However, if the COC has not been taken according to these directions prior to the first missed withdrawal bleed or if two withdrawal bleeds are missed, pregnancy must be ruled out before COC use is continued.
Withdrawal bleeding under treatment with Roxina does not necessarily occur every 4 weeks but at a reduced frequency with intervals of up to 120 days (depending on when the user decides to take a 4-day tablet-free interval). Therefore, the absence of withdrawal bleeding cannot be used as a sign of an unexpected pregnancy and as such, unexpected pregnancy may be difficult to recognize. This may be of particular importance to women using teratogenic drugs. Although pregnancy is unlikely if
Roxina is taken as directed, if for any reason, pregnancy is suspected, a pregnancy test should be performed.
Lactose
The active film-coated tablets contain 46.106 mg lactose (as 48.53 mg of lactose monohydrate). Patients with rare hereditary problems of galactose intolerance, the Lapp-lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Lecithin (soya)
This medicinal product contains 0.070 mg soya lecithin per tablet. Patients with hypersensitivity to peanut or soya should not take this medicine.
4.5 Interaction with other medicinal products and other forms of interaction
Note: The prescribing information of concomitant medications should be consulted to identify potential interactions.
• Effects of other medicinal products on Roxina
Interactions can occur with drugs that induce microsomal enzymes which can result in increased clearance of sex hormones and which may lead to breakthrough bleeding and/or contraceptive failure.
Management
Enzyme induction can already be observed after a few days of treatment. Maximal enzyme induction is generally seen within a few weeks. After the cessation of drug therapy enzyme induction may be sustained for about 4 weeks.
Short-term treatment
Women on treatment with enzyme inducing drugs should temporarily use a barrier method or another method of contraception in addition to the COC. The barrier method must be used during the whole time of the concomitant drug therapy and for 28 days after its discontinuation.
During the period when the barrier method is used, tablet taking should not be interrupted by a tablet-free interval.
Long-term treatment
In women on long-term treatment with hepatic enzyme-inducing active substances, another reliable, nonhormonal, method of contraception is recommended.
The following interactions have been reported in the literature.
Substances increasing the clearance of COCs (diminished efficacy of COCs by enzyme-induction), e.g.:
Barbiturates, bosentan, carbamazepine, phenytoin, primidone, rifampicin, and HIV medication ritonavir, nevirapine and efavirenz and possibly also felbamate, griseofulvin, oxcarbazepine, topiramate and products containing the herbal remedy St. John’s Wort (hypericum perforatum).
Substances with variable effects on the clearance of COCs:
When co-administered with COCs many combinations of HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors, including combinations with HCV inhibitors can increase or decrease plasma concentrations of estrogen or progestins. The net effect of these changes may be clinically relevant in some cases.
Therefore, the prescribing information of concomitant HIV/HCV medications should be consulted to identify potential interactions and any related recommendations. In case of any doubt, an additional barrier contraceptive method should be used by women on protease inhibitor or non-nucleoside reverse transcriptase inhibitor therapy.
Substances decreasing the clearance of COCs (enzyme inhibitors):
The clinical relevance of potential interactions with enzyme inhibitors remains unknown.
Concomitant administration of strong CYP3A4 inhibitors can increase plasma concentrations of the estrogen or the progestin or both.
In a multiple dose study with a drospirenone (3 mg/day) / ethinylestradiol (0.02 mg/day) combination, co-administration of the strong CYP3A4 inhibitor ketoconazole for 10 days increased the AUC(0-24 h) of drospirenone and ethinylestradiol 2.7-fold and 1.4-fold, respectively.
Etoricoxib doses of 60 to 120 mg/day have been shown to increase plasma concentrations of ethinylestradiol 1.4 to 1.6-fold, respectively when taken concomitantly with a combined hormonal contraceptive containing 0.035 mg ethinylestradiol.
• Effects of Roxina on other medicinal products
Oral contraceptives may affect the metabolism of certain other active substances. Accordingly, plasma and tissue concentrations may either increase (e.g. ciclosporin) or decrease (e.g. lamotrigine).
Based on in vitro inhibition studies and in vivo interaction studies in female volunteers using omeprazole, simvastatin and midazolam as marker substrate, an interaction of drospirenone at doses of 3 mg with the metabolism of other active substances is unlikely.
Clinical data suggests that ethinylestradiol is inhibiting the clearance of CYP1A2 substrates leading to a weak (e.g. theophylline) or moderate (e.g. tizanidine) increase in their plasma concentration.
• Other forms of interactions
In patients without renal insufficiency, the concomitant use of drospirenone and ACE-inhibitors or NSAIDs did not show a significant effect on serum potassium. Nevertheless, concomitant use of Roxina with aldosterone antagonists or potassiumsparing diuretics has not been studied. In this case, serum potassium should be tested during the first treatment cycle. See also section 4.4.
• Laboratory tests
The use of contraceptive steroids may influence the results of certain laboratory tests, including biochemical parameters of liver, thyroid, adrenal and renal function, plasma levels of (carrier) proteins, e.g. corticosteroid-binding globulin and lipid/lipoprotein fractions, parameters of carbohydrate metabolism and parameters of coagulation and fibrinolysis. Changes generally remain within the normal laboratory range. Drospirenone causes an increase in plasma renin activity and plasma aldosterone induced by its mild antimineralocorticoid activity.
4.6 Fertility, pregnancy and lactation
Pregnancy
Roxina is not indicated during pregnancy.
The possibility of pregnancy should be considered in any patient who may be experiencing symptoms of pregnancy, especially if she has not adhered to the prescribed schedule. If pregnancy occurs during use of with Roxina, the preparation should be withdrawn immediately. Extensive epidemiological studies have revealed neither an increased risk of birth defects in children born to women who used COCs prior to pregnancy, nor a teratogenic effect when COCs were taken inadvertently during pregnancy.
Animal studies have shown undesirable effects during pregnancy and lactation (see section 5.3). Based on these animal data, undesirable effects due to hormonal action of the active compounds cannot be excluded. However, general experience with COCs during pregnancy did not provide evidence for an actual adverse effect in humans.
The available data regarding the use of Roxina during pregnancy are too limited to permit conclusions concerning negative effects of Roxina on pregnancy, health of the foetus or neonate. To date, no relevant epidemiological data are available.
Withdrawal bleeding under treatment with Roxina normally does not occur every 4 weeks but at a reduced frequency with intervals of up to 120 days. Unexpected pregnancy may be difficult to recognize.
If for any reason pregnancy is suspected in a woman using Roxina, a pregnancy test should be performed.
The increased risk of VTE during the postpartum period should be considered when re-starting Roxina (see section 4.2 and 4.4).
Breastfeeding
Lactation may be influenced by COCs as they may reduce the quantity and change the composition of breast milk. Therefore, the use of COCs should generally not be recommended until the breast-feeding mother has completely weaned her child. Small amounts of the contraceptive steroids and/or their metabolites may be excreted with the milk during COC use. These amounts may affect the child.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. No effects on ability to drive and use machines have been observed in users of COCs.
4.8 Undesirable effects
Summary of safety profile
The most commonly reported adverse reactions with drospirenone/ethinylestradiol (3 mg/0.02 mg) are nausea and breast pain. They occur in > 3% of users.
Serious adverse reactions are arterial and venous thromboembolism, breast cancer and focal nodular hyperplasia.
Tabulated summary of adverse reactions
The frequencies of ADRs of the table below include the labeled ADRs of {Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 24+4-day regimen}, which are combined with the ADRs reported in clinical trials with
{Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 5x24} (such as Roxina) (N=2623). In those cases where ADRs were reported in studies of both clinical development programs, and the frequency under {Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 5x24} was higher, the frequency under {Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 5x24} is shown.
|
System Organ Class |
Frequency of adverse reactions | |||
|
(MedDRA |
Common |
Uncommon |
Rare |
Not known |
|
version 18.0) |
(>1/100 to <1/10) |
(>1/1,000 to <1/100) |
(> 1/10,000 to <1/1,000) |
(cannot be estimated from the available data) |
|
Infections and infestations |
Candidiasis | |||
|
Blood and lymphatic system disorders |
Anaemia, Thrombocythaem ia | |||
|
Immune system disorders |
Allergic reaction |
Hypersensitivit y | ||
|
Endocrine disorders |
Endocrine disorder | |||
|
Metabolism and nutrition disorders |
Increased appetite, Anorexia, Hyperkalaemia, Hyponatraemia | |||
|
Psychiatric disorders |
Emotional lability Depression Libido decreased |
Nervousness, Somnolence |
Anorgasmia, Insomnia | |
|
Nervous system disorders |
Headache |
Dizziness, Paresthesia, |
Vertigo, Tremor | |
|
Eye disorders |
Conjunctivitis, Dry eye, Eye disorder | |||
|
Cardiac disorders |
Tachycardia | |||
|
Vascular disorders |
Migraine |
Varicose vein, Hypertension |
Phlebitis, Vascular disorder, | |
|
Syncope, Venous thromboembolis m (VTE), Arterial thromboembolis m (ATE) | ||||
|
Gastrointestinal disorders |
Nausea |
Abdominal pain, Vomiting, Dyspepsia, Flatulence, Gastritis, Diarrhoea |
Abdomen enlarged, Gastrointestinal disorder, Gastrointestinal fullness, Hiatus hernia, Oral candidiasis, Constipation, Dry mouth | |
|
Hepatobiliary disorders |
Biliary pain, Cholecystitis | |||
|
Skin and subcutaneous tissue disorders |
Acne, Pruritus, Rash |
Chloasma, Eczema, Alopecia, Dermatitis acneiform, Dry skin, Erythema nodosum, Hypertrichosis, Skin disorder, Skin striae, Contact dermatitis, Photosensitive dermatitis, Skin nodule |
Erythema multiforme | |
|
Musculoskeletal and connective tissue disorders |
Back pain, Pain in extremity, Muscle cramps |
|
Reproductive system and breast disorders |
Breast pain, Metrorrhagia*, Amenorrhea |
Vaginal candidiasis, Pelvic pain, Breast enlargement, Fibrocystic breast, Uterine / Vaginal bleeding*, Genital discharge, Hot flushes, Vaginitis, Menstrual disorder, Dysmenorrhea, Hypomenorrhea, Menorrhagia, Vaginal dryness, Papanicolaou smear suspicious |
Dyspareunia, Vulvovaginitis, Postcoital bleeding, Withdrawal bleeding, Breast cyst, Breast hyperplasia, Breast neoplasm, Cervical polyp, Endometrial atrophy, Ovarian cyst, Uterine enlargement | |
|
General disorders and administration site conditions |
Asthenia, Sweating increased, Oedema (Generalized oedema, Peripheral oedema, Face edema) |
Malaise | ||
|
Investigations |
Weight increase |
Weight decrease |
* bleeding irregularities usually subside during continued treatment
For venous thromboembolic events (deep vein thrombosis, pulmonary embolism) and arterial thromboembolic events (myocardial infarction, cerebrovasculair accident), breast cancer, focal nodular hyperplasia (benign liver tumors) and migraine see also sections 4.3 and 4.4.
Description of selected adverse reactions
An increased risk of arterial and venous thrombotic and thrombo-embolic events, including myocardial infarction, stroke, transient ischemic attacks, venous thrombosis and pulmonary embolism has been observed in women using CHCs, which are discussed in more detail in section 4.4.
Adverse reactions with very low frequency or with delayed onset of symptoms which are considered to be related to the group of combined oral contraceptives are listed below (see also sections 4.3 and 4.4:
Tumours
- The frequency of diagnosis of breast cancer is very slightly increased among COC users. As breast cancer is rare in women under 40 years of age the excess number is small in relation to the overall risk of breast cancer. Causation with COC use is unknown.
- Liver tumors (benign and malignant)
Other conditions
- Erythema nodosum, erythema multiforme
- Women with hypertriglyceridemia (increased risk of pancreatitis when using COCs)
- Hypertension
- Occurrence or deterioration of conditions for which association with COC use is not conclusive: jaundice and/or pruritus related to cholestasis; gallstone formation; porphyria; systemic lupus erythematosus; hemolytic uremic syndrome; Sydenham’s chorea; herpes gestationis; otosclerosisrelated-hearing loss
- In women with hereditary angioedema exogenous estrogens may induce or exacerbate symptoms of angioedema
- Liver function disturbances
- Changes in glucose tolerance or effect on peripheral insulin resistance
- Crohn’s disease, ulcerative colitis.
- Chloasma
- Hypersensitivity (including symptoms such as rash, urticaria)
Interactions
Breakthrough bleeding and/or contraceptive failure may result from interactions of other drugs (enzyme inducers, some antibiotics) with oral contraceptives (see section 4.5).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
There has not yet been any experience of overdose with Roxina. On the basis of general experience with combined oral contraceptives, symptoms that may possibly occur in case of taking an overdose of hormone containing tablets are: nausea, vomiting and, in young girls, slight vaginal bleeding. There are no antidotes and further treatment should be symptomatic.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Sex hormones and modulators of the genital system; Progestogens and estrogens, fixed combinations, ATC code: G03AA12
In clinical trials performed with {Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 5x24} (product such as Roxina) in the European Union/Canada and in the USA the following Pearl Indices were calculated:
EU/Canada: Pearl Index for method failure: 0.59 (upper two-sided 95 % confidence limit: 1.22).
Overall Pearl Index (method failure + patient failure): 0.63 (upper two-sided 95% confidence limit: 1.24).
USA: Overall Pearl Index (method failure + patient failure): 1.65 (upper two-sided 95% confidence limit: 2.65).
The contraceptive effect of {Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 5x24} is based on the interaction of various factors, the most important of which are seen as the inhibition of ovulation and the changes in the endometrium.
{Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 5x24} is a COC with ethinylestradiol and the progestogen drospirenone. In a therapeutic dosage, drospirenone also possesses antiandrogenic and mild antimineralocorticoid properties. It has no estrogenic, glucocorticoid and antiglucocorticoid activity. This gives drospirenone a pharmacological profile closely resembling the natural hormone progesterone.
There are indications from clinical studies that the mild antimineralocorticoid properties of {Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 24+4-day regimen} result in a mild antimineralocorticoid effect.
{Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 5x24} is a COC with a flexible extended regimen based on the conventional COC {Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 24+4-day regimen}, therefore the minimum continuous tablet intake is 24 days and the length of the tablet-free interval is 4 days.
A multicenter open randomized parallel group study (EU/Canada) comparing {Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 5x24} with
{Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 24+4-day regimen} demonstrated that the flexible regimen, when used to achieve a maximum length of bleeding-free intervals, was able to reduce the total number of days of menstrual bleeding per year from a mean of 66 days ({Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 24+4-day regimen}) to a mean of 41 days ({Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 5x24}).
Two multicenter, double blind, randomized, placebo controlled studies were performed to evaluate the efficacy and safety of {Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 24+4-day regimen} in women with moderate acne vulgaris.
After six months of treatment, in comparison with placebo, {Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 24+4-day regimen} showed a statistically significantly greater reduction of 15.6% (49.3% versus 33.7%) in inflammatory lesions, 18.5% (40.6% versus 22.1%) in non-inflammatory lesions, and 16.5% (44.6% versus 28.1%) in total lesion counts. In addition, a higher percentage of subjects, 11.8% (18.6 % versus 6.8%), showed a ‘clear’ or ‘almost clear’ rating on the Investigator’s Static Global Assessment (ISGA) scale.
5.2 Pharmacokinetic properties
Drospirenone
Absorption
Orally administered drospirenone is rapidly and almost completely absorbed. Maximum concentrations of the active substance in serum of about 38 ng/ml are reached at about 1-2 h after single ingestion. Bioavailability is between 76 and 85%. Concomitant ingestion of food has no influence on the bioavailability of drospirenone.
Distribution
After oral administration, serum drospirenone levels decrease with a terminal half-life of 31 h. Drospirenone is bound to serum albumin and does not bind to sex hormone binding globulin (SHBG) or corticoid binding globulin (CBG). Only 3-5% of the total serum concentrations of the active substance are present as free steroid. The ethinylestradiol-induced increase in SHBG does not influence the serum protein binding of drospirenone. The mean apparent volume of distribution of drospirenone is 3.7 ± 1.2 l/kg.
Biotransformation
Drospirenone is extensively metabolized after oral administration. The major metabolites in the plasma are the acid form of drospirenone, generated by opening of the lactone ring, and the 4,5-dihydro-drospirenone-3-sulfate, formed by reduction and subsequent sulfatation. Drospirenone is also subject to oxidative metabolism catalyzed by CYP3A4.
In vitro, drospirenone is capable to inhibit weakly to moderately the cytochrome P450 enzymes CYP1A1, CYP2C9, CYP2C19 and CYP3A4.
Elimination
The metabolic clearance rate of drospirenone in serum is 1.5 ± 0.2 ml/min/kg. Drospirenone is excreted only in trace amounts in unchanged form. The metabolites of drospirenone are excreted with the faeces and urine at an excretion ratio of about
1.2 to 1.4. The half-life of metabolite excretion with the urine and faeces is about 40 h.
Steady-state conditions
During a treatment cycle, maximum steady-state concentrations of drospirenone in serum of about 70 ng/ml are reached after about 8 days of treatment. Serum drospirenone levels accumulated by a factor of about 3 as a consequence of the ratio of terminal half-life and dosing interval.
Special Populations
Renal impairment
Steady-state serum drospirenone levels in women with mild renal impairment (creatinine clearance CLcr, 50-80 ml/min) were comparable to those of women with normal renal function. The serum drospirenone levels were on average 37% higher in women with moderate renal impairment (CLcr, 30-50 ml/min) compared to those in women with normal renal function. Drospirenone treatment was also well tolerated by women with mild and moderate renal impairment. Drospirenone treatment did not show any clinically significant effect on serum potassium concentration.
Hepatic impairment
In a single dose study, oral clearance (CL/F) was decreased approximately 50% in volunteers with moderate hepatic impairment as compared to those with normal liver function. The observed decline in drospirenone clearance in volunteers with moderate hepatic impairment did not translate into any apparent difference in terms of serum potassium concentrations. Even in the presence of diabetes and concomitant treatment with spironolactone (two factors that can predispose a patient to hyperkalemia) an increase in serum potassium concentrations above the upper limit of the normal range was not observed. It can be concluded that drospirenone is well tolerated in patients with mild or moderate hepatic impairment (Child-Pugh B).
Ethnic groups
No clinically relevant differences in the pharmacokinetics of drospirenone or ethinylestradiol between Japanese and Caucasian women have been observed.
Ethinylestradiol
Absorption
Orally administered ethinylestradiol is absorbed rapidly and completely. Peak serum concentrations of about 33 pg/ml are reached within 1-2 hours after single oral administration. Absolute bioavailability as a result of presystemic conjugation and first-pass metabolism is approximately 60%. Concomitant intake of food reduced the bioavailability of ethinylestradiol in about 25% of the investigated subjects while no change was observed in the others.
Distribution
Serum ethinylestradiol levels decrease in two phases, the terminal disposition phase is characterized by a half-life of approximately 24 hours. Ethinylestradiol is highly but non-specifically bound to serum albumin (approximately 98.5%), and induces an increase in the serum concentrations of SHBG and corticoid binding globulin (CBG). An apparent volume of distribution of about 5 l/kg was determined.
Biotransformation
Ethinylestradiol is subject to presystemic conjugation in both small bowel mucosa and the liver. Ethinylestradiol is primarily metabolized by aromatic hydroxylation but a wide variety of hydroxylated and methylated metabolites are formed, and these are present as free metabolites and as conjugates with glucuronides and sulfate. The metabolic clearance rate of ethinylestradiol is about 5 ml/min/kg.
In vitro, ethinylestradiol is a reversible inhibitor of CYP2C19, CYP1A1 and CYP1A2 as well as a mechanism based inhibitor of CYP3A4/5, CYP2C8, and CYP2J2.
Elimination
Ethinylestradiol is not excreted in unchanged form to any significant extent. The metabolites of ethinylestradiol are excreted at a urinary to biliary ratio of 4:6. The half-life of metabolite excretion is about 1 day.
Steady-state conditions
Steady-state conditions are reached after approximately 14 days of continued daily tablet intake. Serum levels of ethinylestradiol accumulate by a factor of about 1.5 to 2.3.
5.3 Preclinical safety data
In laboratory animals, the effects of drospirenone and ethinylestradiol were confined to those associated with the recognised pharmacological action. In particular, reproduction toxicity studies revealed embryotoxic and fetotoxic effects in animals which are considered as species specific. At exposures exceeding those in users of {Drospirenone/Ethinylestradiol 3 mg/0.02 mg, 5x24} effects on sexual differentiation were observed in rat fetuses but not in monkeys.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Tablet core:
Lactose monohydrate Maize starch
Maize starch, pregelatinised
Macrogol polyvinyl alcohol grafted copolymer
Magnesium stearate
Film-coating:
Polyvinyl alcohol Titanium dioxide (E171)
Talc
Macrogol 3350 Lecithin (soya)
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
2 years
6.4
Special precautions for storage
Store below 25 °C. Store in the original package in order to protect from light.
The partially used blister should be removed from the etui bag and stored back inside the carton protected from light until ready for use.
6.5 Nature and contents of container
Roxina 0.02/3 mg film-coated tablets are packaged in transparent PVC/PE/PVDC-Aluminum blister packs. The blisters are packed into a cardboard box with a patient information leaflet, an etui storage bag and 5x7 weekdays stickers enclosed in each box.
Pack size:
5x24 film-coated tablets
6.6 Special precautions for disposal
No special requirements for disposal.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Gedeon Richter Plc Gyomroi Utca 19-21 Budapest HU-1103 Hungary
8 MARKETING AUTHORISATION NUMBER(S)
PL 04854/0146
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
14/11/2016
10 DATE OF REVISION OF THE TEXT
14/11/2016
These incidences were estimated from the totality of the epidemiological study data, using relative risks for the different products compared with levonorgestrel-containing CHCs.
Mid-point of range of 5-7 per 10,000 WY, based on a relative risk for CHCs containing levonorgestrel versus non-use of approximately 2.3 to 3.6.