Sayana 104 Mg/0.65 Ml Suspension For Injection
Package leaflet: Information for the user
SAYANA® 104 mg/0.65ml suspension for injection
Medroxyprogesterone acetate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them.
- If you get any side effects, talk to your doctor,pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4
What is in this leaflet:
1. What SAYANA is and what it is used for
2. What you need to know before you use SAYANA
3. How to use SAYANA
4. Possible side effects
5. How to store SAYANA
6. Contents of the pack and other information
1. What SAYANA is and what it is used for
SAYANA is a contraceptive. It can be used:
• For long-term contraception where you and the person who provides your contraception (e.g. your doctor, nurse or healthcare provider) have decided that this method is the most suitable for you. However, if you wish to use SAYANA for more than 2 years, your health professional/doctor/nurse may wish to re-evaluate the risks and benefits of using SAYANA to make sure that it is still the best option for you.
• By teenagers, but only after other methods of contraception have been discussed with the person who provides your contraception and are considered unsuitable or unacceptable.
The active ingredient in SAYANA, medroxyprogesterone acetate (MPA), is similar to (but not the same as) the natural hormone progesterone that is produced in the ovaries during the second half of your menstrual cycle. SAYANA acts by preventing an egg from fully developing and being released from the ovaries during your menstrual cycle. If an egg is not released it cannot become fertilised by sperm and result in pregnancy.
2. What you need to know before you use SAYANA Do not use SAYANA
• If you are allergic (hypersensitive) to medroxyprogesterone acetate (MPA) or any of the other ingredients of SAYANA (listed in section 6)
• If you think you may be pregnant
• If you have unexplained vaginal bleeding
• If you have liver disease
• If you have had or think you have cancer of the breast or sex organs
• If you have a blood clot in a vein in your leg (a "deep vein thrombosis") or a blood clot that has travelled to your lung or another part of your body (an "embolus")
• If you have problems with your circulation (e.g. pains in your legs or chest when you walk), or with your blood clotting too easily ('thrombosis' or 'embolism')
• If you have problems due to the metabolism of your bones
• If you have or have had a disease affecting the blood vessels of the brain
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using SAYANA
Before your doctor prescribes SAYANA, you may need to have a physical examination.
It is important to tell your doctor if you have, or have had in the past, any of the following conditions.
Your doctor will then discuss with you whether SAYANA is suitable for you.
Tell your doctor if you have:
• Migraine headaches
• Diabetes or a family history of diabetes
• Severe pain or swelling in the calf (indicating a possible clot in the leg, which may be called phlebitis)
• A blood clot in the lung (pulmonary embolism)
• A blood clot in your eye affecting your vision (retinal thrombosis)
• A history of heart disease or cholesterol problems including any family history
• If you have recently had a “hydatidiform mole” which is a type of abnormal pregnancy
• Past history of depression
• Irregular, light, or heavy menstrual periods
• An unusual breast x-ray, fibrocystic breast disease, breast nodules or lumps, or bleeding from your nipples
• Had a stroke
• Family history of breast cancer
• Kidney disease
• High blood pressure
• Asthma
• Epilepsy
Protection against sexually transmitted diseases
SAYANA does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Other medicines and SAYANA
Please tell your doctor, pharmacist or nurse if you are taking or have recently taken any other medicines, including medicines obtained without a prescription. There are some medicines that may interact with SAYANA, which include medicines that thin your blood (anticoagulants).
Medicines can sometimes interfere with each other. If you receive treatment from any other doctor, nurse or qualified healthcare professional make sure they are aware that you are using SAYANA as a contraceptive.
Do not take SAYANA if you are pregnant. If you think you may have become pregnant while using SAYANA tell your doctor immediately.
If you are breast-feeding, the injection should be given no sooner than 6 weeks after childbirth, when the baby is more developed. SAYANA can be passed to the nursing infant in the breast milk, however no harmful effects have been found in children.
Always ask your doctor, nurse, or healthcare professional for advice before taking any medicine.
Driving and using machines
No effects on the ability to drive or use machines have been seen with SAYANA.
SAYANA contains methyl parahydroxybenzoate (E218) & propyl parahydroxybenzoate (E216) and sodium
Methyl parahydroxybenzoate (E218) & propyl parahydroxybenzoate (E216). These may cause allergic reactions (possibly delayed).
This medicinal product contains less than 1 mmol sodium (23 mg) per 104 mg/0.65 ml, i.e. essentially ‘sodium-free’.
3. How to use SAYANA Method and route of administration
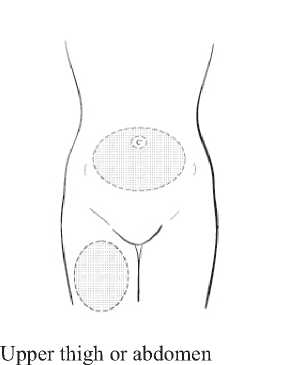
SAYANA is injected under the skin into the front upper thigh or abdomen. The injection should be administered by your doctor, nurse, or healthcare provider. The detailed instructions on the injection procedure provided at the end of this leaflet should be followed. You should continue to receive SAYANA for as long as instructed by your doctor.
First injection
A dose of 104 mg of SAYANA is given subcutaneously (just under the skin), into the front upper thigh or abdomen every 3 months (12 to 13 weeks). SAYANA will only be effective if you receive your injection at the proper time. To ensure that you are not pregnant at the time of the first injection, it is essential that your first injection be given ONLY during the first 5 days of your normal menstrual cycle.
After childbirth: If you use SAYANA after having a baby and you are not breast-feeding, the first injection MUST be given within 5 days.
There is evidence that women prescribed SAYANA immediately after childbirth or termination of pregnancy can experience prolonged and heavy bleeding. Because of this, SAYANA should be used with caution at this time.
Further injections
Further doses of SAYANA will then be given every 12 to 13 weeks, (but no later than 14 weeks past your last injection), regardless of when and how much menstrual bleeding you have.
It is important that you receive your next injections at the right time.
If you miss an injection of SAYANA
If you miss your injection or wait longer than 14 weeks between injections, there is a greater risk that you could become pregnant. Tell your doctor, pharmacist, or healthcare professional to find out when you should receive your next injection of SAYANA and which type of contraception should be used in the meantime.
Switching from other Methods of Contraception
When you switch from other contraceptive methods, your doctor will make sure you are not at risk of becoming pregnant by giving you your first injection at the appropriate time. If you switch from oral contraceptives, you should have your first injection of SAYANA within 7 days after taking your last pill.
What if you decide you want to get pregnant
Your usual level of fertility will return when the effect of the last injection has worn off. The time this takes varies in different women, and does not depend on how long you have been using SAYANA. In most women the effect will have worn off 5 to 6 months after the last injection. Over 80 % of women will get pregnant within a year of stopping SAYANA. It is possible to get pregnant in the first month after missing an injection.
Possible side effects
4.
Like all medicines, SAYANA can cause side effects, although not everybody gets them. If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
If you experience any of the following serious side effects, seek medical help immediately:
• A serious allergic reaction (it is not known how frequently this occurs)
Symptoms include sudden wheeziness, difficulty in breathing or dizziness, swelling of the eyelids, face, lips or throat, skin rash, hives.
• A blood clot in the lungs (it is not known how frequently this occurs)
Symptoms include
o an unusual sudden cough (which may bring up blood) o severe pain in the chest which may increase with deep breathing o sudden unexplained breathlessness or rapid breathing o severe light headedness or dizziness o rapid or irregular heartbeat o severe pain in your abdomen
• A blood clot in the leg (it is not known how frequently this occurs)
Symptoms include severe pain or swelling in either of your legs or feet that may be accompanied by tenderness, warmth or discoloured skin
• A blood clot in the eye (it is not known how frequently this occurs)
Symptoms include loss of vision, pain and swelling of the eye especially if sudden
• A stroke (it is not known how frequently this occurs)
Symptoms include
o weakness or numbness of the face, arm or leg, especially on one side of the body o sudden confusion, trouble speaking or understanding o sudden trouble seeing in one or both eyes
o sudden trouble walking, dizziness, loss of balance or coordination o sudden, severe or prolonged headache with no known cause o loss of consciousness or fainting with or without seizure.
Other side effects include:
Common: (may affect up to 1 in 10 people)
• Weight increase
• Abdominal pain (cramps)
• Nausea
• Acne
• Amenorrhea (very light or no period)
• Heavy, frequent and/or unexpected bleeding
• Irregular periods
• Period pains
• Breast pain / tenderness
• Depression
• Weakness or tiredness
• Headache
• Injection site reactions (including pain, tenderness, lump, persistent skin indentation/dimpling)
• Irritability
• Anxiety
• Difficulty sleeping
• Decreased sexual feeling
• Vaginal irritation or itching
• Mood changes
• Dizziness
• Back pain
• Pain in limbs
• Abnormal cervical smear
Uncommon: (may affect up to 1 in 100 people)
• Drug allergy
• Hirsutism (abnormal hairiness)
• Feeling bloated
• Fluid retention
• Vaginal discharge
• Vaginal dryness
• Pain during sexual intercourse
• Ovarian cyst
• Pelvic pain
• Premenstrual syndrome
• Change in breast size
• Milky discharge from breasts in women who are not breast-feeding
• Change in appetite
• Muscle cramps
• Joint pain
• Sleepiness
• Migraine
• Vertigo (a spinning sensation)
• Hot flushes
• High blood pressure
• Rapid heart rate
• Varicose veins
• Rash
• Itching
• Hives
• Hair loss
• Skin irritation
• Bruising
• Facial discoloration
• Inflammation in the veins (felt as tenderness or redness in the affected area)
• Nervousness
• Loss of bone mineral density (a test used to diagnose osteoporosis or weak bones)
• Weakness
• Decreased glucose tolerance (excess sugar level in the blood)
• Emotional disturbance Inability to achieve a sexual climax
Rare: (may affect up to 1 in 1,000 people)
• Breast cancer
• FeverWeight decrease
• Deformation of skin at the injection site
• Abnormal liver function test results (blood tests to measure liver injury)
Not known: frequency cannot be estimated from the available data
• Osteoporosis (weak bones) including osteoporotic fractures
• Seizures
• Abnormal liver function such as yellowing of the skin or eyes (jaundice)
• Skin stretch mark
Possible effect on your periods
Most women using SAYANA will experience a change in their bleeding patterns. It is likely that fewer women will experience irregular bleeding, and after 12 months of use 60% will experience little or no bleeding at all.
Possible effect on your bones
SAYANA works by lowering levels of estrogen and other hormones. However, low estrogen levels can cause bones to become thinner (by reducing bone mineral density). Women who use SAYANA tend to have lower bone mineral density than women of the same age who have never used it. The effects of SAYANA are greatest in the first 2-3 years of use. Following this, bone mineral density tends to stabilise and there appears to be some recovery when SAYANA is stopped. It is not yet possible to say whether SAYANA increases the risk of osteoporosis (weak bones) and fractures in later life.
The following are risk factors in the development of osteoporosis in later life. You should discuss with your doctor before starting treatment if you have any of the following as an alternative contraceptive may be more suitable to your needs;
• Chronic alcohol and/or tobacco use
• Chronic use of drugs that can reduce bone mass, e.g. epilepsy medication or steroids
• Low body mass index or eating disorder, e.g. anorexia nervosa or bulimia
• Previous low trauma fracture that was not caused by a fall
• Strong family history of osteoporosis
Teenagers (up to 18 years) Normally, the bones of teenagers are rapidly growing and increasing in strength. The stronger the bones are when adulthood is reached, the greater the protection against osteoporosis in later life. Since SAYANA may cause teenage bones to become thinner at a time when they should be growing, its effect may be particularly important in this age group. Bones start to recover when SAYANA is stopped, but it is not yet known whether the bone mineral density reaches the same levels as it would have if SAYANA had never been used.
You should therefore discuss whether another form of contraception might be more suitable for you with the person who provides your contraception before starting SAYANA.
If you use SAYANA, it may help your bones if you take regular weight-bearing exercise and have a healthy diet, including an adequate intake of calcium (e.g. in dairy products) and vitamin D (e.g. in oily fish).
Possible risk of cancer
Studies of women who have used a range of medicine based contraception found that women who used injectable progestogen like SAYANA for contraception had no increased overall risk of developing cancer of the ovary, womb, cervix, or liver.
Breast cancer is rare under 40 years of age, but the risk increases as a woman becomes older.
There seems to be a slightly increased risk of breast cancer in women who take injectable contraceptives compared to women of the same age who do not use hormonal contraceptives.
This small extra risk of developing breast cancer has to be weighed against the known benefits of medicines like SAYANA. It is not certain whether the injection causes the increased risk of breast cancer. It may be that women receiving the injection are examined more often, so that breast cancer is noticed earlier. The breast cancer seems less likely to have spread when found in women who receive medicines like SAYANA than in women who do not.
The risk of finding breast cancer is not affected by how long a woman is on the injection, but by the age at which she stops. This is because the risk of breast cancer strongly increases as a woman becomes older. Ten years after stopping hormonal contraceptive injections, the risk of finding breast cancer is the same as for women who have never used hormonal contraceptives.
In 10,000 women who receive injections like SAYANA for up to 5 years, but stop taking it by the time they are aged 20, it is estimated that less than 1 additional case of breast cancer would be found up to 10 years afterwards, compared with the number found in 10,000 women who had never had the injection.
For 10,000 women who are on injections like SAYANA for 5 years and stop it by the age of 30, there would be 2 or 3 extra cases of breast cancer found up to 10 years afterwards (in addition to the 44 cases of breast cancer found in 10,000 women in this age group who had never had the injection).
For 10,000 women who take SAYANA for 5 years and stop it by the age of 40, there would be about 10 extra cases found up to 10 years afterwards (in addition to 160 cases of breast cancer found in 10,000 women in this age group who had never had the injection).
Other risks:
If you develop
• A sudden partial or complete loss of vision, double vision, blood clotting disorders such as pulmonary
embolus (blood clot in the lung) or a stroke you should not receive further injections of SAYANA
• Migraine you should consult your doctor before receiving further injections of SAYANA
• Jaundice (yellowing of the skin or eyes) you should consult your doctor before receiving further
injections of SAYANA
Reporting of side effects
If you get any side effects talk to you doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store SAYANA
• Keep out of sight and reach of children.
• Do not refrigerate or freeze.
• Do not use SAYANA after the expiry dates stated on the pre-filled syringe and the carton after EXP. The expiry date refers to the last day of that month.
• Once opened: use immediately.
Carefully dispose of any SAYANA suspension that has not been injected. The syringe and needle should NEVER be reused. Any unused product should be disposed of safely after use, in accordance with local guidance for the disposal of sharps.
6. Contents of the pack and other information
What SAYANA contains
The active substance is medroxyprogesterone acetate (MPA).
The pre-filled syringe of SAYANA contains 104mg medroxyprogesterone acetate (MPA) in 0.65 ml.
The other ingredients are macrogol, methyl parahydroxybenzoate (E 218), propyl parahydroxybenzoate (E 216), sodium chloride, polysorbate 80, monobasic sodium phosphate monohydrate, disodium phosphate dodecahydrate, methionine, povidone, sodium hydroxide and/or hydrochloric acid for pH adjustment and water for injection.
What SAYANA looks like and contents of the pack
SAYANA is a white to off-white suspension for subcutaneous injection (an injection given under the skin). It is supplied in a pre-filled syringe with a rubber tip cap.
SAYANA is available in pack sizes of one pre-filled syringe with one 26G 3/8” needle and of six pre-filled syringes with six 26G, 3/8” needles.
Not all pack sizes may be sold.
Marketing Authorisation Holder
Pfizer Limited Ramsgate Road Sandwich Kent
CT13 9NJ United Kingdom
Manufacturer
Pfizer Manufacturing Belgium NV Rijksweg 12 B-2870 Puurs Belgium
For any information about this medicinal product, please contact the local representative of the Marketing Authorisation Holder:
United Kingdom
Pfizer Limited Ramsgate Road Sandwich, CT13 9NJ-United Kingdom Tel: + 44 (0) 1304 616161
This medicinal product is authorised in the Member States of the EEA under the following names:
|
Austria |
SAYANA 104 mg/0,65 ml |
|
Belgium |
SAYANA 104 mg/0,65 ml |
|
Czech Republic |
SAYANA 104 mg/0,65 ml |
|
Germany |
SAYANA 104 mg/0,65 ml |
|
Hungary |
SAYANA 104 mg/0,65 ml |
|
Netherlands, United Kingdom |
Sayana |
This leaflet was last revised in 12/2015
Ref: SN 4 0
m SAYANA®
104mg
MEDROXYPROGESTERONE ACETATE 104mg/0.65m suspension for injection
The following information is intended for medical or healthcare professionals only:
SAYANA 104 mg/0.65 ml suspension for injection medroxyprogesterone acetate
INSTRUCTIONS FOR ADMINISTRATION: PREPARING AND GIVING A SUBCUTANEOUS INJECTION OF SAYANA
Introduction
SAYANA should be administered by a person (such as your doctor, nurse or healthcare professional) trained in administering subcutaneous injections.
The following instructions explain how to prepare and inject SAYANA. The instructions should be read carefully and followed step-by-step.
The injection should not be mixed with any other medicine.
Instructions for Administration of SAYANA for Subcutaneous Use Getting ready
Do not refrigerate. Ensure that the medication is at room temperature prior to injection (to ensure appropriate viscosity of the suspension). Make sure the following components (Diagrams 1 and 2) are available.
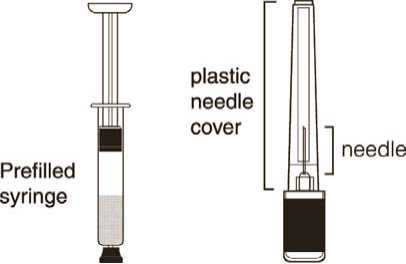
Diagram 1 Diagram 2
SAYANA, as with other parenteral drug products, should be inspected visually for particulate matter and discoloration prior to administration.
Step 1: Choosing and preparing the injection area.
Choose the injection area in either the upper thigh or abdomen, see shaded areas (Diagram 3). Avoid bony areas and the umbilicus.
Diagram 3
Use an alcohol pad to wipe the skin in the injection area you have chosen. Allow the skin to dry.
Step 2: Syringe preparation
Gently twist off the protective end cap from the needle to break the seal (Diagram 4). Set aside.
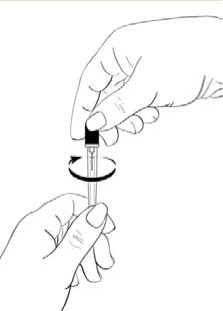
Diagram 4
While holding the syringe firmly by the barrel pointing upward, shake it vigorously for at least 1 minute to thoroughly mix the medication (Diagram 5).

Then remove the protective cap from the tip of the syringe barrel. Diagram 5
While holding the syringe barrel, attach the needle to the barrel of the syringe firmly by pushing the plastic needle cover down fully with a slight twisting movement (Diagram 6).

Diagram 6
While continuing to hold the syringe barrel firmly, remove the protective plastic needle cover from the needle without twisting, making sure the needle is still firmly attached to the syringe (Diagram 7).
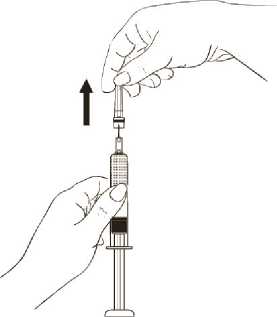
Diagram 7
While holding the syringe with the needle pointing upward, gently push in the plunger until the medicine is up to the top of the syringe. There should be no air within the barrel (Diagram 8).

Diagram 8
Step 3: Injecting the dose.
Gently grasp and squeeze a large area of skin in the chosen injection area between the thumb and fore-finger, pulling it away from the body. Insert the needle at a 45 degree angle so that most of the needle is in the fatty tissue. The plastic hub of the needle should be nearly or almost touching the skin (Diagram 9).
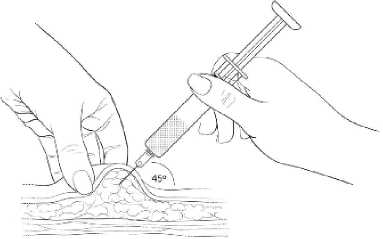
Diagram 9
Inject the medication slowly until the syringe is empty (Diagram 10). . This should take about 5-7 seconds.
. It is important that the entire dose of Sayana is given

Inject slowly (5-7 seconds)
Diagram 10
When the entire dose is completely injected, gently pull the needle out of the skin.
Use a clean cotton pad to press lightly on the injection area for a few seconds. Do NOT rub the area.
DISPOSING OF SUPPLIES
The syringe and needle should NEVER be reused.
Any unused product should be disposed of safely after use, in accordance with local guidance for the disposal of sharps.
Ref: SN 4 0
SN4_0 Page 14 of 14 2015-0013068