Sodium Chloride 0.9% Solution For Injection
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Sodium Chloride 0.9% solution for injection
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Sodium chloride 0.9%w/v
Electrolytes:
Na+ 154 mmol/l
Cl- 154 mmol/l
For a full list of excipients, see section 6.1
3. PHARMACEUTICAL FORM
Solution for Injection A clear colourless solution
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
Sodium Chloride 0.9% solution for injection is used as a solvent and carrier solution for injectable drugs.
4.2 Posology and method of administration
Posology As required
Method of administration
Intravenous, intramuscular or subcutaneous use
4.3 Contraindications
None stated.
4.4 Special warnings and precautions for use
If Sodium Chloride 0.9% solution for injection is to be administered subcutaneously be aware that any additions to the isotonic normal saline solution could render it hypertonic and thus cause pain at the injection site.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed.
4.6 Fertility, pregnancy and lactation
None stated
4.7 Effects on ability to drive and use machines
No studies on the ability to drive and use machines have been performed.
4.8 Undesirable effects
None stated.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
No particulars stated.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
ATC code: B05XA03
Sodium chloride 0.9% solution shows the same osmotic pressure as body fluids.
The isotonic solution of sodium chloride is a suitable vehicle for administration of a large number of drugs and electrolytes.
5.2
Pharmacokinetic properties
Sodium and chloride electrolytes distribute, primarily, in the extracellular fluid. Because the physiological saline solution is isotonic, its administration will not produce a change in the osmotic pressure of the extracellular fluid, whereby water will not pass into the intracellular compartment and both ions will practically enter the cell.
5.3 Preclinical safety data
Safety of sodium chloride isotonic solutions is adequately recognized in the fluid therapy field worldwide, thanks to a wide experience available in the use of this solution as a restorer of the hydro-electrolytic balance.
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Water for Injections
Sodium hydroxide for pH adjustment.
Hydrochloric acid for pH adjustment.
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
a) Shelf life of the product as packaged for sale:
Shelf life of the pharmaceutical product: 2 years
b) Shelf life after first opening of the container :
The product should be used immediately after opening.
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5 Nature and contents of container
Low density polyethylene ampoules (LDPE).
- Carton with 20 ampoules of 5 ml
- Carton with 20 ampoules of 10 ml
- Carton with 20 ampoules of 20 ml
6.6 Special precautions for disposal and other handling
It is not necessary to sterilise the bottle before its opening.
It is not necessary to use any cutting element to open the ampoule.
Once the ampoule is opened the top of it can perfectly be adjusted to the syringe cone (cone Luer), with which it is necessary to use the needle.
Handling instructions
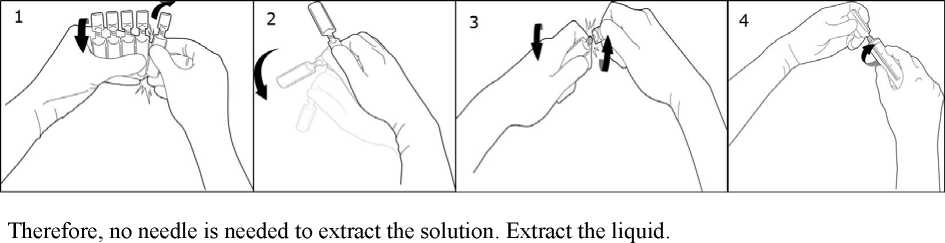
To break off a single ampoule, twist one ampoule against the remaining ampoules of the pack without touching the head and neck of the ampoules (1). Shake the ampoule with one single movement as shown below in order to remove the liquid kept in the cap (2). To open the ampoule, twist the ampoule body and the ampoule head in opposite directions until the neck breaks off (3). Connect the ampoule to the luer-syringe or luer-lock syringe as shown in figure (4).
The solution does not contain any type of preservative or bactericide, so open and unused ampoules should be discarded immediately.
7. MARKETING AUTHORISATION HOLDER
Fresenius Kabi Limited Cestrian Court Eastgate Way Manor Park Runcorn Cheshire WA7 1NT UK
8.
MARKETING AUTHORISATION NUMBER
PL 08828/0178
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
30/08/2007
10 DATE OF REVISION OF THE TEXT
06/09/2016