Spiriva 18 Microgram Inhalation Powder Hard Capsules
Out of date information, search anotherCommon:
Uncommon:
Rare:
Not known:
Spiriva® 18 microgram inhalation powder, hard capsules
_(tiotropium)_
PATIENT INFORMATION LEAFLET
Your medicine is available using the name Spiriva 18 microgram Inhalation Powder, Hard Capsules but will be referred to as Spiriva throughout this leaflet.
Read all of this leaflet carefully before you start taking this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Spiriva is and what it is used for
2. Before you take Spiriva
3. How to take Spiriva
4. Possible side effects
5. How to store Spiriva
6. Further information
1. WHAT SPIRIVA IS AND WHAT IT IS USED FOR
Spiriva helps people who have chronic obstructive pulmonary disease (COPD) to breathe more easily. COPD is a chronic lung disease that causes shortness of breath and coughing. The term COPD is associated with the conditions chronic bronchitis and emphysema. As COPD is a chronic disease you should take Spiriva every day and not only when you have breathing problems or other symptoms of COPD.
Spiriva is a long-acting bronchodilator that helps to open your airways and makes it easier to get air in and out of the lungs. Regular use of Spiriva can also help you when you have on-going shortness of breath related to your disease and will help you to minimise the effects of the disease on your everyday life. It also helps you to be active longer. Daily use of Spiriva will also help to prevent sudden, short-term worsening of your COPD symptoms which may last for several days. The effect of this medicine lasts for 24 hours, so you only need to take it once a day. For correct dosing of Spiriva please see section 3 HOW TO TAKE SPIRIVA and the instructions for use provided on the other side of the leaflet.
2. BEFORE YOU TAKE SPIRIVA
Please read the following questions carefully. If you can answer any of these questions with "Yes" please discuss this with your doctor before taking Spiriva
• are you allergic to tiotropium, atropine or similar drugs such as ipratropium or oxitropium or to milk protein?
• are you taking any other medicinal products containing ipratropium or oxitropium?
• are you pregnant, do you think you are pregnant, or are you breast-feeding?
• are you suffering from narrow angle glaucoma, prostate problems or have difficulty passing urine?
• do you have any kidney problems?
Do not take Spiriva
You should not use Spiriva if you are allergic (hypersensitive) to tiotropium or to lactose monohydrate which contains milk protein.
You should also not use Spiriva if you are allergic (hypersensitive) to atropine or substances related to it e.g. ipratropium or oxitropium.
You should not use Spiriva if you are under 18 years of age.
Take special care with Spiriva
• If you suffer from narrow angle glaucoma, prostate problems or have difficulty passing urine.
• If you have problems with your kidneys.
• Spiriva is indicated for maintenance treatment of your chronic obstructive pulmonary disease, it should not be used to treat a sudden attack of breathlessness or wheezing.
• Immediate allergic reactions such as rash, swelling, itching, wheezing or breathlessness may occur after administration of Spiriva. If this occurs, please consult your doctor immediately.
• Inhaled medicines such as Spiriva may cause tightness of the chest, coughing, wheezing or breathlessness immediately after inhalation. If this occurs, please consult your doctor immediately.
• Take care not to let the inhalation powder enter your eye as this may result in precipitation or worsening of narrow-angle glaucoma, which is a disease of the eyes. Eye pain or discomfort, blurred vision, seeing halos around lights or coloured images in association with red eyes may be signs of an acute attack of narrow-angle glaucoma. Eye symptoms may be accompanied by headache, nausea or vomiting. You should stop using tiotropium bromide and immediately consult your doctor, preferably an eye specialist, when signs and symptoms of narrow-angle glaucoma appear.
• Dry mouth, which has been observed with anti-cholinergic treatment, may in the long term be associated with dental caries. Therefore, please remember to pay attention to oral hygiene.
• Do not take Spiriva more frequently than once daily.
Taking other medicines
Please inform your doctor or pharmacist if you are taking, or have recently taken, any other medicines, even those not prescribed.
Please tell your doctor or pharmacist if you are taking/have taken similar medicines for your lung disease, such as ipratropium or oxitropium.
No specific side effects have been reported when Spiriva has been taken together with other products used to treat COPD such as reliever inhalers e.g. salbutamol, methylxanthines e.g. theophylline and/or oral and inhaled steroids e.g. prednisolone.
Pregnancy and breast-feeding
If you are pregnant or believe you are pregnant, or if you are breast-feeding, consult with your doctor. You should not use this medicine unless specifically recommended by your doctor.
Driving and using machines
The occurrence of dizziness, blurred vision, or headache may influence the ability to drive and use machinery.
Important information about some of the ingredients of Spiriva
When taken according to dosage recommendations, one capsule once a day, each dose supplies up to 5.5mg lactose monohydrate.
3. HOW TO TAKE SPIRIVA
Follow your doctor's instructions about when and how to take your medicine. If you are unsure ask your doctor or pharmacist.
The recommended dose is to inhale the contents of 1 capsule (18 micrograms of tiotropium) once a day. Do not take more than the recommended dose.
You should try to take the capsule at the same time every day. This is important because Spiriva is effective over 24 hours.
Do not swallow the capsules.
The HandiHaler device, which you should put the Spiriva capsule into, makes holes in the capsule and allows you to breathe in the powder.
Make sure that you have a HandiHaler and that you can use it properly. The instructions for use of the HandiHaler are provided on the other side of this leaflet.
Make sure that you do not blow into the HandiHaler.
If you have any problems using the HandiHaler, ask your doctor, nurse or pharmacist to show you how it works.
You should clean your HandiHaler once a month. Cleaning instructions for the HandiHaler are provided on the other side of this leaflet.
When taking Spiriva, take care not to let any of the powder enter your eyes. If any powder does get into your eyes you may get blurred vision, eye pain and/or red eyes, you should wash your eyes in warm water immediately. Then talk to your doctor immediately for further advice.
If you feel that your breathing is worsening, you should tell your doctor as soon as possible.
If you take more Spiriva than you should
If you inhale from more than 1 capsule of Spiriva in a day, you should talk to your doctor immediately. You may be at a higher risk of experiencing a side effect such as dry mouth, constipation, difficulties passing urine, increased heart beat, or blurred vision.
If you forget to take Spiriva
If you forget to take a dose, take one as soon as you remember but do not take two doses at the same time or on the same day. Then take your next dose as usual.
If you stop taking Spiriva
Before you stop taking Spiriva, you should talk to your doctor or your pharmacist. If you stop taking Spiriva the signs and symptoms of COPD may worsen.
If you have any further questions on the use of this product, ask your doctor.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Spiriva can cause side effects, although not everybody gets them. Evaluation of the side effects is based on the following frequencies:
affects 1 to 10 patients in 100
affects 1 to 10 patients in 1,000
affects 1 to 10 patients in 10,000
frequency cannot be estimated from the available data
The side effects described below have been experienced by people taking this medicine and they are listed according to frequency as either common, uncommon, rare or not known.
Common:
• Dry mouth: this is usually mild
Uncommon:
• Dizziness
• Headache
• Taste disorders
• Blurred vision
• Irregular heart beat (atrial fibrillation)
• Sore throat (pharyngitis)
• Hoarseness (dysphonia)
• Cough
• Heart burn (gastrooesophageal reflux disease)
• Constipation
• Fungal infections of the oral cavity and throat (oropharyngeal candidiasis)
• Rash
• Difficulty passing urine (urinary retention)
• Painful urination (dysuria)
Rare:
• Difficulty in sleeping (insomnia)
• Seeing halos around lights or coloured images in association with red eyes (glaucoma)
• Increase of the measured eye pressure
• Irregular heart beat (supraventricular tachycardia)
• Faster heart beat (tachycardia)
• Feeling your heartbeat (palpitations)
• Tightness of the chest, associated with coughing, wheezing or breathlessness immediately after inhalation (bronchospasm)
• Nosebleed (epistaxis)
• Inflammation of the larynx (laryngitis)
• Inflammation of the sinuses (sinusitis)
• Blockage of intestines or absence of bowel movements (intestinal obstruction including ileus paralytic)
• Inflammation of the gums (gingivitis)
• Inflammation of the tongue (glossitis)
• Difficulties swallowing (dysphagia)
• Inflammation of the mouth (stomatitis)
• Feeling sick (nausea)
• Allergic reactions (hypersensitivity), including immediate reactions
• Serious allergic reaction which causes swelling of the face or throat (angioedema)
• Nettle rash (urticaria)
• Itching (pruritus)
• Infections of the urinary tract
Not known:
• Depletion of body water (dehydration)
• Tooth decay (dental caries)
• Infections or ulcerations of the skin
• Dryness of the skin
• Swelling of joints
Serious side effects include allergic reactions which cause swelling of the face or throat (angioedema) or tightness of the chest, associated with coughing, wheezing or breathlessness immediately after inhalation (bronchospasm). If this occurs, please consult your doctor immediately.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE SPIRIVA
• Keep out of the sight and reach of children.
• Do not use Spiriva after the expiry date which is stated on the carton and the blister foil.
The expiry date refers to the last day of that month.
• Do not store above 25°C. Do not freeze.
• Once you have taken your first capsule of Spiriva from the blister strip you must continue to take the capsules for the next 9 days, one capsule a day, from the same strip.
• If your capsule appears to be discoloured or show any sign of deterioration, you should ask your pharmacist who will advice you what to do.
• If you have any leftover/unused capsules, please return this to your pharmacist for safe disposal.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What Spiriva contains
Each capsule contains 18 micrograms of the active ingredient tiotropium (as bromide monohydrate).
During inhalation, 10 micrograms tiotropium are delivered from the mouthpiece of the HandiHaler®.
The other ingredient is lactose monohydrate.
What Spiriva looks like and contents of the pack
Spiriva is a light green hard capsule containing a white or yellowish white powder, with product code printed in black 'Ti 01' on the cap and company logo on the body. The HandiHaler device is light grey with 'HandiHaler' printed on the front. The HandiHaler device comprises of a dust cap, mouthpiece, piercing button and centre chamber.
The HandiHaler is the device, which must be used with Spiriva.
Your medicine is available in packs containing 30 capsules and 1 HandiHaler device.
Manufacturer
The manufacturer of Spiriva is: Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim am Rhein, Germany.
Procured from within the EU and repackaged by: Doncaster Pharmaceuticals Group Ltd., Kirk Sandall, Doncaster, DN3 1QR.
Product Licence holder: BR Lewis Pharmaceuticals Ltd., Kirk Sandall, Doncaster, DN3 1QR.
PL No: 08929/0414 | POM |
Leaflet revision and issue date (Ref): 28.02.13
Spiriva® and HandiHaler® are registered trademarks of Boehringer Ingelheim Pharma GmbH & Co. KG.
Dear Patient,
The HandiHaler enables you to inhale the medicine contained in the Spiriva capsule - that your physician has prescribed for your breathing problems.
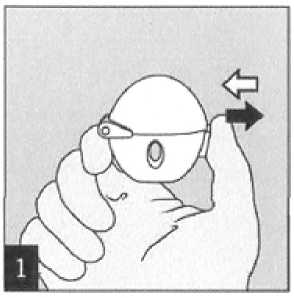
Remember to carefully follow your doctor's instructions for using Spiriva. The HandiHaler is especially designed for Spiriva. You must not use it to take any other medication. You can use your HandiHaler for up to one year to take your medication.
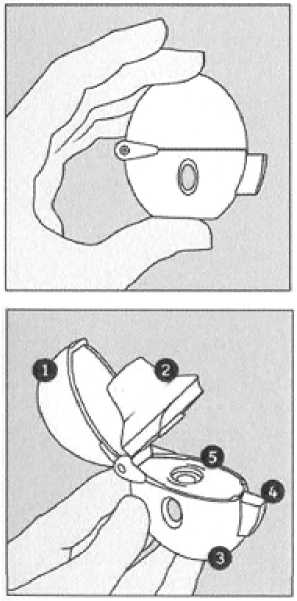
The HandiHaler
|
1. |
Dust cap |
|
2. |
Mouth piece |
|
3. |
Base |
|
4. |
Piercing button |
|
5. |
Centre chamber |
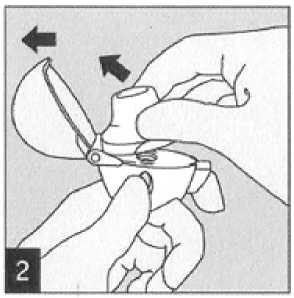
To release the dust cap press the piercing button completely in and let go.
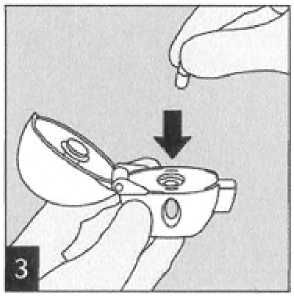
Open the dust cap completely by pulling it upwards. Then open the mouthpiece by pulling it upwards.
Remove a Spiriva capsule from the blister (only immediately before use, see blister handling) and place it in the centre chamber (5), as illustrated. It does not matter which way the capsule is placed in the chamber.
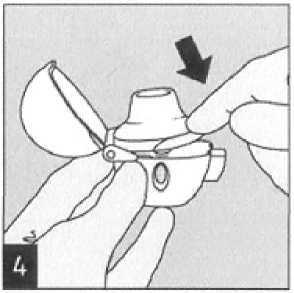
Close the mouthpiece firmly until you hear a click, leaving the dust cap open.
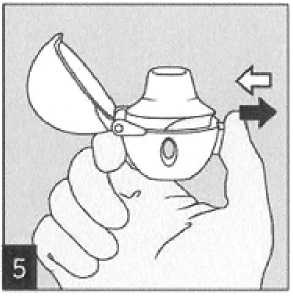
Hold the HandiHaler device with the mouthpiece upwards and press the piercing button completely in only once, and release. This makes holes in the capsule and allows the medication to be released when you breathe in.
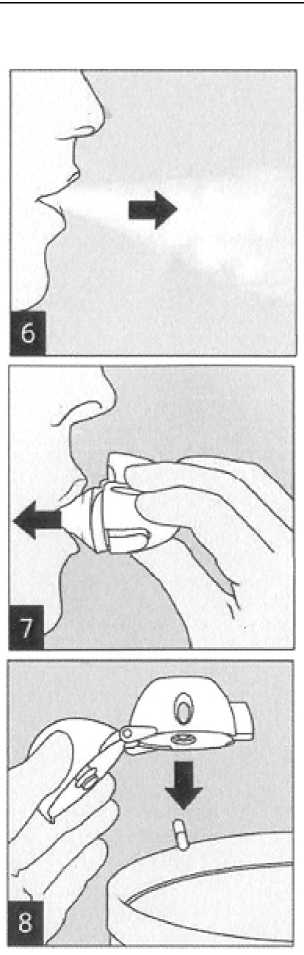
Breathe out completely.
Important:
Please avoid breathing into the mouthpiece at any time.
Open the mouthpiece again. Tip out the used capsule and dispose. Close the mouthpiece and dust cap for storage of your HandiHaler device.
Raise the HandiHaler to your mouth and close your lips tightly around the mouthpiece. Keep your head in an upright position and breathe in slowly and deeply but at a rate sufficient to hear or feel the capsule vibrate. Breathe until your lungs are full; then hold your breath as long as comfortable and at the same time take the HandiHaler out of your mouth. Resume normal breathing. Repeat steps 6 and 7 once, in order to empty the capsule completely.
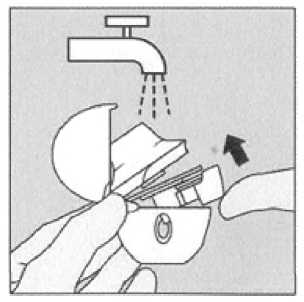
Blister handling
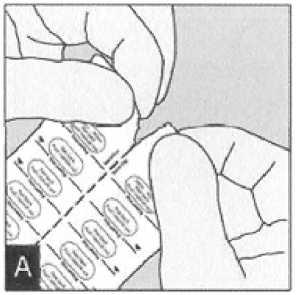
Separate the blister strips by tearing along the perforation.
Cleaning your HandiHaler:
Clean the HandiHaler once a month. Open the dust cap and mouthpiece. Then open the base by lifting the piercing button. Rinse the complete inhaler with warm water to remove any powder. Dry the HandiHaler thoroughly by tipping excess of water out on a paper towel and air-dry afterwards, leaving the dust cap, mouthpiece and base open. It takes 24 hours to air dry, so clean it right after you have used it and it will be ready for your next dose. If needed the outside of the mouthpiece may be cleaned with a moist but not wet tissue.
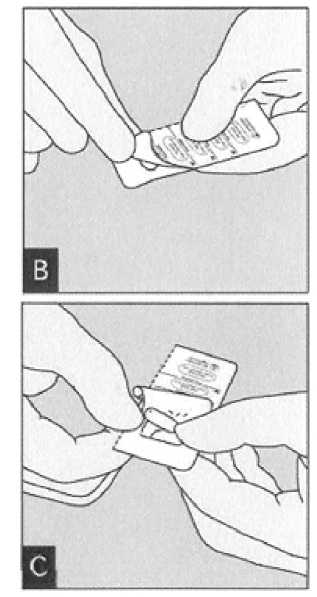
Peel back foil (only immediately before use) using the tab until one capsule is fully visible.
In case a second capsule is exposed to air inadvertently this capsule has to be discarded.
Remove capsule.
Spiriva capsules contain only a small amount of powder so that the capsule is only partially filled.
Leaflet revision and issue date (Ref): 28.02.13
Spiriva® and HandiHaler® are registered trademarks of Boehringer Ingelheim Pharma GmbH & Co. KG.
Page 2 of 2