Timolol Eye Drops Bp 0.25% W/V
Out of date information, search anotherTIMOLOL EYE DROPS BP
0.25% AND 0.5% W/V
PACKAGE LEAFLET: INFORMATION FOR THE USER
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you..
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Timolol is and what it is used for
2. What you need to know before you take Timolol
3. How to take Timolol
4. Possible side effects
5. How to store Timolol
6. Contents of the pack and other information
1. What timolol is and what it is used for
Your eye drops are called Timolol Eye Drops BP. Timolol is one of a group of drugs called beta-blockers. It reduces the pressure in your eyes.
Timolol eye drops BP are used to treat conditions such as glaucoma, which cause a raised pressure in your eyes.
You can only get Timolol Eye Drops BP with a prescription from your doctor.
2. What you need to know before you take Timolol Do not take Timolol if you:
• if you are allergic to timolol, beta-blockers or any of the other ingredients of this medicine (listed in section 6)..
• if you have now or have had in past respiratory problems such as asthma, severe chronic obstructive bronchitis (severe lung disease which may cause wheeziness, difficulty in breathing and/or longstanding cough).
• have heart disease which gives you a slow pulse rate.
Don’t wear soft contact lenses while you are using these eye drops. You can put your contact lenses back in 15 minutes after using your eye drops. But make sure you take your lenses out before you put the next dose of eye drops in your eyes.
Warnings and precautions:
Before you use this medicine, tell your doctor or pharmacist or nurse if you have now or have had in the past
• coronary heart disease (symptoms can include chest pain or tightness, breathlessness or choking), heart failure, low blood pressure, disturbances of heart rate such as slow heart beat.
• breathing problems, asthma or chronic obstructive pulmonary disease
• poor blood circulation disease (such as Raynaud’s disease or Raynaud’s syndrome)
• diabetes as Timolol may mask signs and symptoms of low blood sugar
• over activity of the thyroid gland as Timolol may mask signs and symptoms
Tell your doctor before you have an operation that you are using Timolol as Timolol may change effects of some medicines used during anaesthesia.
Children:
Timolol eye drop solution should generally be used with caution in young patients. In newborns, infants and younger children Timolol should be used with extreme caution. If coughing, wheezing, abnormal breathing or abnormal pauses in breathing (apnoea) occur; the use of the medication should be stopped immediately. Contact your doctor as soon as possible. A portable apnoea monitor may also be helpful.
Timolol eye drop solution has been studied in infants and children aged 12 days to 5 years, who have raised pressure in the eye(s) or have been diagnosed with glaucoma. For more information, talk to your doctor.
Other medicines and Timolol
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines:
Timolol can affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Tell your doctor if you are using or intend to use medicines to lower blood pressure, heart medicine or medicines to treat diabetes. Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Quinidine (used to treat heart conditions and some types of malaria), antidepressants known as fluoxetine and paroxetine.
Pregnancy, breast-feeding and fertility
Do not use Timolol if you are pregnant unless your doctor considers it necessary.
Do not use Timolol if you are breast-feeding. Timolol may get into your milk. Ask your doctor for advice before taking any medicine during breast-feeding.
Effects on the ability to drive or to use machines
You may find that your vision is blurred after you put the eye drops into your eyes or you may have difficulty in seeing at night or when the light is dim. Do not drive or use machinery until your vision is completely normal.
3. How to take Timolol
Always use Timolol eye drops solution exactly as your doctor or pharmacist has told you. You should check with your doctor or pharmacist if you are not sure.
These eye drops are sterile while the bottle is sealed. Do not use them if the bottle is open or the seal is broken when you get them.
The label will tell you what to do if you can’t remember. You should use the drops twice a day at first. The dose will depend on conditions, how quickly you respond to the medicine and whether you are taking any other treatment. The first dose is usually one drop of 0.25% w/v solution twice a day. Some people need a stronger dose and others need a weaker one. Your doctor will change your treatment as necessary. Use the drops until your doctor says you no longer need them.
• First wash your hands.
• Twist off the protective cap from the bottle
• Tilt your head backwards
• Pull your bottom eyelid downwards to make a pocket
• Hold the bottle in your hand between your thumb and first finger, with the tip close to your bottom eyelid.
• Squeeze the bottle gently until one drop of the solution falls into your eye
• Blink a few times to spread the eye drop
• Repeat the process with your other eye if your doctor tells you to treat both eyes
• Put the cap back on the bottle
• Wash your hands again.
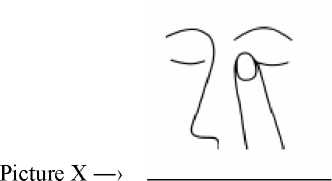
After using Timolol, press a finger into the corner of your eye, by the nose (picture X) for 2 minutes. This helps to stop Timolol getting into the rest of the body.
Use in children:
A detailed medical examination should precede the use of Timolol. Your doctor will carefully evaluate the risks and benefits when considering treatment with Timolol. If the benefits outweigh the risks, it is recommended to use the lowest active agent concentration available once daily.
With regard to “the use in children”, the 0,1% active agent concentration may be sufficient to control pressure within the eye. If the pressure is not sufficiently controlled with this dosage, a twice daily application at 12-hourly intervals may be necessary. Patients, especially newborn, should be closely observed for one to two hours after the first dose and careful monitoring for adverse events should be carried out until surgery is performed.
Route and/or method of administration (picture X)
One drop only of Timolol should be instilled per dosing time.
After instillation keep the eyes closed for as long as possible (e.g. 3 - 5 minutes) and apply pressure to the corner of the eye closest to the nose to prevent Timolol eye drops spreading throughout the body.
Duration of treatment .
For a transient treatment in the paediatric population.
If you take more Timolol than you should
If you use too many eye drops, rinse your eyes immediately with clean water. If you swallow the eye drops, go to your nearest hospital causality department or doctor immediately.
Please take this leaflet, any remaining eye drops and the container with you to the hospital or doctor so that they know which eye drops were consumed.
These eye drops are only for you. Only a doctor can proscribe them for you. Never give them to anyone else. If you forget to take Timolol
If you forget to use your eye drops, use them as soon as you remember and then carry on as normal.
Do not take a double dose to make up a forgotten dose.
If you stop taking Timolol
Don’t stop using the eye drops without talking to your doctor first. You may not feel any different, but the drops are helping to reduce the pressure in your eyes. If you are not sure about anything, ask your doctor or pharmacist or nurse.
4. Possible side effects
Like all medicines, Timolol eye drops can cause side effects, although not everybody gets them.
You can usually carry on taking the drops, unless the effects are serious. If you're worried, talk to a doctor or pharmacist. Do not stop using timolol without speaking to your doctor.
Like other medicines applied into eyes, timolol is absorbed into the blood. This may cause similar side effects as seen with ‘intravenous' and/or 'oral' as applicable beta-blocking agents. Incidence of side effects after topical ophthalmic administration is lower than when medicines are, for example, taken by mouth or injected. Listed side effects include reactions seen within the class of beta-blockers when used for treating eye conditions:
Allergic condition which causes joint pain, skin rashes and fever (Systemic lupus erythematosus), generalized allergic reactions including swelling beneath the skin that can occur in areas such as the face and limbs, and can obstruct the airway which may cause difficulty swallowing or breathing, hives or itchy rash, localized and generalized rash, itchiness, severe sudden life-threatening allergic reaction.
Low blood glucose levels.
Difficulty sleeping (insomnia), depression, nightmares, memory loss.
Fainting, stroke, reduced blood supply to the brain, increases in signs and symptoms of myasthenia gravis (muscle disorder), dizziness, unusual sensations like pins and needles, and headache.
Signs and symptoms of eye irritation (e.g. burning, stinging, itching, tearing, redness), inflammation of the eyelid, inflammation in the cornea, blurred vision and detachment of the layer below the retina that contains blood vessels following filtration surgery which may cause visual disturbances, decreased corneal sensitivity, dry eyes, corneal erosion (damage to the front layer of the eyeball), drooping of the upper eyelid (making the eye stay half closed), double vision.
Slow heart rate, chest pain, palpitations, oedema (fluid build up), changes in the rhythm or speed of the heartbeat, congestive heart failure (heart disease with shortness of breath and swelling of the feet and legs due to fluid build up), a type of heart rhythm disorder, heart attack, heart failure.
Low blood pressure, Raynaud's phenomenon, cold hands and feet. Constriction of the airways in the lungs (predominantly in patients with pre-existing disease), difficulty breathing, cough.
Taste disturbances, nausea, indigestion, diarrhoea, dry mouth, abdominal pain, vomiting.
Hair loss, skin rash with white silvery coloured appearance (psoriasiform rash) or worsening of psoriasis, skin rash.
Muscle pain not caused by exercise.
Sexual dysfunction, decreased libido.
Muscle weakness/tiredness.
In very rare cases, some patients with severe damage to the clear layer at the front of the eye (the cornea) have developed cloudy patches on the cornea due to calcium build-up during treatment.
If any of the side effects get serious, or if you notice any side effects not mentioned in this leaflet, please, tell your doctor or pharmacist.
Your doctor may want to examine your eyes regularly to see if the medicine is working.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Timolol
Keep this medicine out of the sight and reach of children.
Do not let anyone else use your eye drops. Keep them in safe place, out of the sunlight, where children cannot see or reach them.
Do not use the eye drops after the bottle has been open for a month. Get a new bottle. Do not use this medicine after the expiry date which is stated on the bottle after EXP. The expiry date refers to the last day of the month.
Don’t keep any eye drops you no longer need, unless your doctor tells you to. Give them back to your pharmacists.
Do not throw away any medicines via waste water or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect environment.
6. Contents of the pack and other information What Timolol eye drops contain:
Each bottle contains 5ml of a solution which has Timolol Maleate Ph Eur as the active ingredient.
The solution also contains: potassium dihydrogen phosphate, disodium hydrogen phosphate, benzalkonium chloride and purified water.
What Timolol eye drops look like and contents of the pack:
Timolol Eye drops BP come in two strengths-0.25% and 0.5% w/v Marketing Authorisation Holder
Norton Healthcare Ltd, Ridings Point, Whistler Drive, Castleford, West Yorkshire, WF10 5HX Manufacturer
Martindale Pharmaceuticals, Bampton Road, Harold Hill, Romford, Essex, RM3 8UG and Medevale Pharma services Ltd, Vale of Bardsley, Aston-under-lyne, Lancs, OL7 9RR.
This leaflet was last revised: Dec 2014 PL 00530/0490-0491