Tiotropium 2.5 Microgram Inhalation Solution
POM
Q Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Evaluation of the side effects is based on the following frequencies:
Common: may affect up to 1 in 10 people Uncommon: may affect up to 1 in 100 people Rare: may affect up to 1 in 1,000 people
Not known: frequency cannot be estimated from the available data
The side effects described below have been experienced by people taking this medicine and they are listed according to frequency as either common, uncommon, rare or not known
|
Side effect |
Frequency COPD |
Frequency Asthma |
|
Dry mouth: this is usually mild |
Common |
Common |
|
Dizziness |
Uncommon |
Uncommon |
|
Headache |
Uncommon |
Uncommon |
|
Difficulty in sleeping (insomnia) |
Rare |
Uncommon |
|
Irregular heart beat (atrial fibrillation, supraventricular tachycardia) |
Rare |
Not known |
|
Feeling your heartbeat (palpitations) |
Rare |
Uncommon |
|
Faster heart beat (tachycardia) |
Rare |
Not known |
|
Cough |
Uncommon |
Uncommon |
|
Nosebleed (epistaxis) |
Rare |
Not Known |
|
Inflammation of the throat (pharyngitis) |
Uncommon |
Uncommon |
|
Hoarseness (dysphonia) |
Uncommon |
Uncommon |
|
Tightness of the chest, associated with coughing, wheezing or breathlessness immediately after inhalation (bronchospasm) |
Rare |
Uncommon |
|
Constipation |
Uncommon |
Rare |
|
Fungal infections of the oral cavity and throat (oropharyngeal candidiasis) |
Uncommon |
Uncommon |
|
Difficulties swallowing (dysphagia) |
Rare |
Not known |
|
Rash |
Uncommon |
Rare |
|
Itching (pruritus) |
Uncommon |
Rare |
|
Difficulties passing urine (urinary retention) |
Uncommon |
Not known |
|
Painful urination (dysuria) |
Uncommon |
Not known |
|
Seeing halos around lights or coloured images in association with red eyes (glaucoma) |
Rare |
Not known |
|
Increase of the measured eye pressure |
Rare |
Not known |
|
Blurred vision |
Rare |
Not know |
|
Inflammation of the larynx (laryngitis) |
Rare |
Not known |
|
Heart burn (gastrooesophageal reflux disease) |
Rare |
Not Known |
|
Dental caries |
Rare |
Not known |
|
Inflammation of the gums (gingivitis) |
Rare |
Rare |
|
Inflammation of the tongue (glossitis) |
Rare |
Not known |
|
Inflammation of the mouth (stomatitis) |
Not known |
Rare |
|
Serious allergic reaction which causes swelling of the mouth and face or throat (angioneurotic oedema) |
Rare |
Rare |
|
Nettle rash (urticaria) |
Rare |
Rare |
|
Infections or ulcerations of the skin |
Rare |
Not known |
|
Dryness of the skin |
Rare |
Not known |
|
Hypersensitivity, including immediate reactions |
Not known |
Rare |
|
Infections of the urinary tract |
Rare |
Not known |
|
Depletion of body water (dehydration) |
Not known |
Not known |
|
Inflammation in sinuses (sinusitis) |
Not known |
Not known |
|
Blockage of intestines or absence of bowel movements (intestinal obstruction, including ileus paralytic) |
Not known |
Not known |
|
Feeling sick (nausea) |
Not known |
Not known |
|
Severe allergic reaction (anaphylactic reaction) |
Not known |
Not known |
|
Swelling of joint |
Not known |
Not known |
Immediate allergic reactions such as rash, nettle rash (urticaria), swelling of the mouth and face or sudden difficulties in breathing (angioneurotic oedema) or other hypersensitivity reactions (such as sudden reduction of your blood pressure or dizziness) may occur individually or as part of severe allergic reaction (anaphylactic reaction) after administration of Spiriva Respimat. If any of these occur, please consult your doctor immediately
In addition, in common with all inhaled medicines, some patients may experience an unexpected tightness of the chest, coughing, wheezing or breathlessness immediately after inhalation (bronchospasm).
Reporting of side effects:
If you get any side effects, talk to your doctor or pharmacist.This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme (Tel: Freephone 0808 100 3352 or Website: www.mhra.aov.uk/vellowcard1. By reporting side effects you can help provide more information on the safety of this medicine.
O How to store Spiriva Respimat
Keep out of the sight and reach of children.
Do not use Spiriva Respimat after the expiry date which is stated on the carton and on the inhaler label. The expiry date refers to the last day of the month. Spiriva Respimat inhaler should be discarded at the latest 3 months after first use (see Instructions for Use overleaf).
Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
If your medicine becomes discoloured or show any other signs of deterioration, ask your pharmacist who will advise you what to do.
O Contents of the pack and other information
What Spiriva Respimat contains:
The active substance is tiotropium. The delivered dose is 2.5 micrograms tiotropium (as bromide monohydrate) per puff (2 puffs comprise one medicinal dose).
The other ingredients are: Benzalkonium chloride, disodium edetate, purified water, and hydrochloric acid 3.6% for pH adjustment.
What Spiriva Respimat looks like and contents of the pack
The Cardboard carton contains one inhaler and one aluminium cylindrical cartridge containing a clear colourless solution for inhalation. A plastic cap is present on both ends of the inhaler: a green cap covering the mouth piece; and a transparent cap where the cartridge is to be inserted. The inhaler has an indicator on the side showing the number of doses left.
Single pack: 1 Respimat inhaler and 1 cartridge, providing 60 puffs (30 medicinal doses)
Manufacturer and Licence Holder
This medicine is manufactured by Boehringer Ingelheim Pharma GmbH &
Co. KG, Binger Strasse 173, D-55216 Ingelhein am Rhein, Germany and is procured from within the EU. Product Licence Holder: Lexon UK Limited, Unit 18 Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 ORE.
If you have any questions or are not sure about anything, ask your doctor or pharmacist. They will have additional information about this medicine and will be able to advise you.
Spiriva® Respimat® is a registered trademark of Boehringer ingelheim Pharma GmbH & Co. KG.
PL 15184/1619 - Spiriva Respimat 250 microgram, Inhalation solution
Leaflet revision date: 04/04/16
Blind or partially sighted? Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
Spiriva® Respimat® 2.5 meg, inhalation solution
(tiotropium bromide)
Patient Information Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Your medicine is called Spiriva Respimat 2.5 meg inhalation solution and will be referred to as Spiriva Respimat throughout the rest of this leaflet.
What is in this leaflet
Q What Spiriva Respimat is and what it is used for Q What you need to know before you take Spiriva Respimat Q How to take Spiriva Respimat Q Possible side effects Q How to store Spiriva Respimat Q Contents of the pack and other information
Q What Spiriva Respimat is and what it is used for
Spiriva Respimat helps people who have chronic obstructive pulmonary disease (COPD) or asthma to breathe more easily. COPD is a long-term lung disease that causes shortness of breath and coughing. The term COPD is associated with the conditions chronic bronchitis and emphysema. Asthma is a long-term disease characterised by airway inflammation and narrowing of the airways. As COPD and asthma are long-term diseases you should take Spiriva Respimat every day and not only when you have breathing problems or other symptoms. When used to treat asthma you should use Spiriva Respimat in addition to so-called inhaled corticosteroids and long-acting IJ2 agonists.
Spiriva Respimat is a long-acting bronchodilator that helps to open your airways and makes it easier to get air in and out of the lungs. Regular use of Spiriva Respimat can also help you when you have on-going shortness of breath related to your disease, and will help to minimise the effects of the disease on your everyday life. Daily use of Spiriva Respimat will also help to prevent any sudden, short-term worsening of your COPD symptoms which may last for several days.
For correct dosing of Spiriva Respimat please see section 3. How to take Spiriva Respimat and the instructions for use provided on the other side of the leaflet.
Q What you need to know before you take Spiriva Respimat
Please read the following questions carefully. If you can answer any of these questions with ‘Yes’ please discuss this with your doctor before taking Spiriva Respimat
• are you allergic (hypersensitive) to tiotropium, atropine or similar drugs such as ipratropium or oxitropium?
• are you taking any other medicinal products containing ipratropium or oxitropium?
• are you pregnant, do you think you are pregnant, or are you breast-feeding?
• are you suffering from blurred vision, eye pain and/or red eyes, prostate problems or have difficulty passing urine?
• do you have any kidney problems?
• have you suffered from a myocardial infarction during the last 6 months or from any unstable or life threatening irregular heart beat or severe heart failure within the past year?
Do not use Spiriva Respimat
• if you are allergic (hypersensitive) to tiotropium, its active ingredient or any of the other ingredients of this medicine (listed in section 6)
• if you are allergic (hypersensitive) to atropine or substances related to it, e.g. ipratropium or oxitropium
Warnings and precautions
Talk to your doctor before taking Spiriva Respimat.
When taking Spiriva Respimat take care not to let any spray enter your eyes. This may result in eye pain or discomfort, blurred vision, seeing halos around lights or coloured images in association with red eyes (i.e. narrow angle glaucoma). Eye symptoms may be accompanied by headache, nausea or vomiting. Wash your eyes in warm water, stop using tiotropium bromide and immediately consult your doctor for further advice.
If your breathing has got worse or if you experience rash, swelling or itching directly after using your inhaler, stop using it and tell your doctor immediately.
Dry mouth which has been observed with anti-cholinergic treatment may in the long term be associated with dental caries. Therefore, please remember to pay attention to oral hygiene.
Spiriva Respimat is indicated for the maintenance treatment of your chronic obstructive pulmonary disease or asthma. Do not use this medicine to treat a sudden attack of breathlessness or wheezing. Your doctor should have given you another inhaler (“rescue medication”) for this.
Please follow the instructions your doctor has given you.
If you have been prescribed Spiriva Respimat for your asthma it should be added on to inhaled corticosteroids and long-acting IJ2 agonists. Continue taking the inhaled corticosteroids as prescribed by your doctor, even if you feel better.
In case you have suffered from a myocardial infarction during the last 6 months or from any unstable or life threatening irregular heart beat or severe heart failure within the past year, please, inform your doctor. This is important to decide if Spiriva is the right medicine for you to take.
Do not take Spiriva Respimat more frequently than once daily.
You should also contact your doctor if you feel that your breathing is worsening.
If you have cystic fibrosis, tell your doctor because Spiriva Respimat could make your cystic fibrosis symptoms worse.
Children and adolescents
Spiriva Respimat is not recommended for children and adolescents under 18 years.
Other medicines and Spiriva Respimat
Please tell your doctor or pharmacist if you are taking, or have recently taken, any other medicines, including medicines obtained without a prescription.
In particular, please tell your doctor or pharmacist if you are taking/have taken anticholinergic drugs, e.g. ipratropium or oxitropium.
No interaction side effects have been reported when Spiriva Respimat has been taken with other products used to treat COPD such as reliever inhalers (e.g. salbutamol), methylxanthines (e.g. theophylline), antihistamines, mucolytics (e.g. ambroxol), leukotriene modifiers (e.g. montelukast), cromones, anti-lgE treatment (e.g. omalizumab) and/or inhaled or oral steroids (e.g. budesonide, prednisolone).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. You should not use this medicine unless specifically recommended by your doctor.
Driving and using machines
No studies on the effects and the ability to drive and use machines have been performed. In case dizziness or blurred vision occurs the ability to drive and use machinery may be influenced.
Q How to take Spiriva Respimat
Always take this medicine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Spiriva Respimat is for inhalation use only.
The recommended dose for adults is:
Spiriva Respimat is effective for 24 hours so you will need to use Spiriva Respimat only ONCE A DAY, if possible at the same time of the day. Each time you use it take TWO PUFFS.
As COPD and asthma are long-term diseases take Spiriva Respimat every day and not only when you experience breathing problems. Do not take more than the recommended dose.
Spiriva Respimat is not recommended for use in children and adolescents below 18 years due to lack of data on safety and efficacy.
Make sure that you know how to use your Spiriva Respimat inhaler properly. The instructions for use of the Spiriva Respimat inhaler are provided on the other side of this leaflet.
If you take more Spiriva Respimat than you should
If you take more Spiriva Respimat than two puffs in one day talk to your doctor immediately. You may be at a higher risk of experiencing a side effect such as dry mouth, constipation, difficulties passing urine, increased heart beat or blurred vision.
If you forget to take Spiriva Respimat
If you forget to take your daily dose (TWO PUFFS ONCE A DAY), don’t worry. Take it as soon as you remember but do not take two doses at the same time or on the same day. Then take your next dose as usual.
If you stop taking Spiriva Respimat
Before you stop taking Spiriva Respimat, you should talk to your doctor or your pharmacist. If you stop taking Spiriva Respimat the signs and symptoms of COPD may worsen.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
CAP
SAFETY CATCH —
CLEAR BASE

W. I
Prepare for first use
Instructions For Use
Introduction
Spiriva Respimat (tiotropium bromide). Read these Instructions for Use before you start using Spiriva Respimat.
You will need to use this inhaler only ONCE A DAY. Each time you use it take TWO PUFFS.
MOUTHPIECE-
AIR VENT-
DOSE-RELEASE BUTTON
PIERCING ELEMENT
CARTRIDGE
• If Spiriva Respimat has not been used for more than 7 days release one puff towards the ground.
• If Spiriva Respimat has not been used for more than 21 days repeat steps 4 to 6 under ‘Prepare for first Use’ until a cloud is visible. Then repeat steps 4 to 6 three more times.
• Do not touch the piercing element inside the clear base.
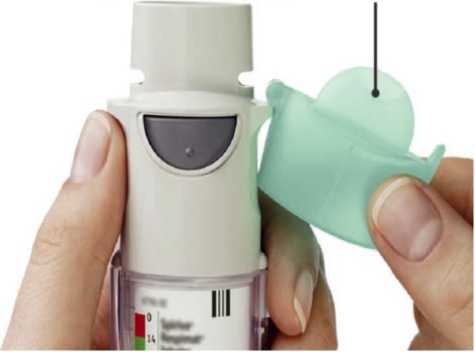
Remove clear base 1- • Keep the cap closed.
• Press the safety catch while firmly pulling off the clear base with your other hand
SAFETY CATCH
CLEAR BASE

Insert cartridge
2. • Insert the narrow end of the cartridge into the inhaler.
• Place the inhaler on a firm surface and push down firmly until it clicks into place.

Replace clear base
3. • Put the clear base back into place until it clicks.
How to care for your Spiriva Respimat
CLEAR BASE
Clean the mouthpiece including the metal part inside the mouthpiece with a damp cloth or tissue only, at least once a week.
Any minor discoloration in the mouthpiece does not affect your Spiriva Respimat inhaler performance. If necessary, wipe the outside of your Spiriva Respimat inhaler with a damp cloth.
When to get a new Spiriva Respimat
Turn
DOSE
INDICATOR

EMPTY
FULL
Keep the cap closed.
Turn the clear base in the direction of the arrows on the label until it clicks (half a turn).
ARROWS

Open
• Your Spiriva Respimat inhaler contains 60 puffs (30 doses) if used as indicated (two puffs/once daily).
• The dose indicator shows approximately how much medication is left.
• When the dose indicator enters the red area of the scale you need to get a new prescription; there is approximately medication for 7 days left
(14 puffs).
• Once the dose indicator reaches the end of the red scale, your Spiriva Respimat locks automatically - no more doses can be released.
At this point, the clear base cannot be turned any further.
• Spiriva Respimat should be discarded three months after you have prepared it for first use, even if it has not been fully used or used at all.
5. • Open the cap until it snaps fully open.
CAP

Press 6- • Point the inhaler toward the ground.
• Press the dose-release button
• Close the cap
• Repeat steps 4-6 until a cloud is visible.
• After a cloud is visible, repeat steps 4-6 three more times.
Your inhaler is now ready to use. These steps will not affect the number of doses available.After preparation your inhaler will be able todeliver 60 puffs (30 doses).
DOSE
RELEASE
BUTTON

Answers to Common Questions
Daily use
TURN
• Keep the cap closed.
• TURN the clear base in the direction of the arrows on the label until it clicks (half a turn).

OPEN
• OPEN the cap until it snaps fully open.
CAP
PRESS
• Breathe out slowly and fully.
• Close your lips around the mouthpiece without covering the air vents. Point your Inhaler to the back of your throat.
• While taking a slow, deep breath through your mouth, PRESS the dose-release button and continue to breathe in slowly for as long as comfortable.
• Hold your breath for 10 seconds or for as long as comfortable.
• Repeat TURN, OPEN, PRESS for a total of 2 puffs.
• Close the cap until you use your inhaler again.
It is difficult to insert the cartridge deep enough.
Did you accidentally turn the clear base before inserting the cartridge?
Open the cap, press the dose-release button, then insert the cartridge.
Did you insert the cartridge with the wide end first?
Insert the cartridge with the narrow end first.
The dose indicator on the Spiriva Respimat reaches zero too soon.
Did you use Spiriva Respimat as indicated (two puffs/Once daily)?
Spiriva Respimat will last 30 days if used at two puffs once daily.
Did you turn the clear base before you inserted the cartridge?
The dose indicator counts each turn of the clear base regardless whether a cartridge has been inserted or not.
Did you spray in the air often to check whether the Spiriva Respimat is working?
Once you have prepared Spiriva Respimat, no test-spraying is required if used daily.
Did you insert the cartridge into a used Spiriva Respimat?
Always insert a new cartridge into a NEW Spiriva Respimat
I cannot press the dose-release button.
Did you turn the clear base?
If not, turn the clear base in a continuous movement until it clicks (half a turn).
Is the dose indicator on the Spiriva Respimat pointing to zero?
The Spiriva Respimat inhaler is locked after 60 puffs (30 medicinal doses). Prepare and use your new Spiriva Respimat inhaler.
My Spiriva Respimat sprays automatically.
Was the cap open when you turned the clear base?
Close the cap, then turn the clear base.
Did you press the dose-release button when turning the clear base?
Close the cap, so the dose-release button is covered, then turn the clear base.
Did you stop when turning the clear base before it clicked?
Turn the clear base in a continuous movement until it clicks (half a turn).
I cannot turn the clear base.
Did you turn the clear base already?
If the clear base has already been turned, follow steps “OPEN” and “PRESS” under “Daily Use” to get your medicine.
Is the dose indicator on the Spiriva Respimat pointing to zero?
The Spiriva Respimat inhaler is locked after 60 puffs (30 medicinal doses). Prepare and use your new Spiriva Respimat inhaler.
My Spiriva Respimat doesn’t spray.
Did you insert a cartridge?
If not, insert a cartridge.
Did you repeat TURN, OPEN, PRESS less than three times after inserting the cartridge?
Repeat TURN, OPEN, PRESS three times after inserting the cartridge as shown in the steps 4 to 6 under “Prepare for first Use”.
Is the dose indicator on the Spiriva Respimat pointing to 0?
If the dose indicator points to 0, you have used up all your medication and the inhaler is locked.
Once your Spiriva Respimat is assembled, do not remove the clear base or the cartridge. Always insert a new cartridge into a NEW Spiriva Respimat.
POM
Q Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Evaluation of the side effects is based on the following frequencies:
Common: may affect up to 1 in 10 people Uncommon: may affect up to 1 in 100 people Rare: may affect up to 1 in 1,000 people
Not known: frequency cannot be estimated from the available data
The side effects described below have been experienced by people taking this medicine and they are listed according to frequency as either common, uncommon, rare or not known
|
Side effect |
Frequency COPD |
Frequency Asthma |
|
Dry mouth: this is usually mild |
Common |
Common |
|
Dizziness |
Uncommon |
Uncommon |
|
Headache |
Uncommon |
Uncommon |
|
Difficulty in sleeping (insomnia) |
Rare |
Uncommon |
|
Irregular heart beat (atrial fibrillation, supraventricular tachycardia) |
Rare |
Not known |
|
Feeling your heartbeat (palpitations) |
Rare |
Uncommon |
|
Faster heart beat (tachycardia) |
Rare |
Not known |
|
Cough |
Uncommon |
Uncommon |
|
Nosebleed (epistaxis) |
Rare |
Not Known |
|
Inflammation of the throat (pharyngitis) |
Uncommon |
Uncommon |
|
Hoarseness (dysphonia) |
Uncommon |
Uncommon |
|
Tightness of the chest, associated with coughing, wheezing or breathlessness immediately after inhalation (bronchospasm) |
Rare |
Uncommon |
|
Constipation |
Uncommon |
Rare |
|
Fungal infections of the oral cavity and throat (oropharyngeal candidiasis) |
Uncommon |
Uncommon |
|
Difficulties swallowing (dysphagia) |
Rare |
Not known |
|
Rash |
Uncommon |
Rare |
|
Itching (pruritus) |
Uncommon |
Rare |
|
Difficulties passing urine (urinary retention) |
Uncommon |
Not known |
|
Painful urination (dysuria) |
Uncommon |
Not known |
|
Seeing halos around lights or coloured images in association with red eyes (glaucoma) |
Rare |
Not known |
|
Increase of the measured eye pressure |
Rare |
Not known |
|
Blurred vision |
Rare |
Not know |
|
Inflammation of the larynx (laryngitis) |
Rare |
Not known |
|
Heart burn (gastrooesophageal reflux disease) |
Rare |
Not Known |
|
Dental caries |
Rare |
Not known |
|
Inflammation of the gums (gingivitis) |
Rare |
Rare |
|
Inflammation of the tongue (glossitis) |
Rare |
Not known |
|
Inflammation of the mouth (stomatitis) |
Not known |
Rare |
|
Serious allergic reaction which causes swelling of the mouth and face or throat (angioneurotic oedema) |
Rare |
Rare |
|
Nettle rash (urticaria) |
Rare |
Rare |
|
Infections or ulcerations of the skin |
Rare |
Not known |
|
Dryness of the skin |
Rare |
Not known |
|
Hypersensitivity, including immediate reactions |
Not known |
Rare |
|
Infections of the urinary tract |
Rare |
Not known |
|
Depletion of body water (dehydration) |
Not known |
Not known |
|
Inflammation in sinuses (sinusitis) |
Not known |
Not known |
|
Blockage of intestines or absence of bowel movements (intestinal obstruction, including ileus paralytic) |
Not known |
Not known |
|
Feeling sick (nausea) |
Not known |
Not known |
|
Severe allergic reaction (anaphylactic reaction) |
Not known |
Not known |
|
Swelling of joint |
Not known |
Not known |
Immediate allergic reactions such as rash, nettle rash (urticaria), swelling of the mouth and face or sudden difficulties in breathing (angioneurotic oedema) or other hypersensitivity reactions (such as sudden reduction of your blood pressure or dizziness) may occur individually or as part of severe allergic reaction (anaphylactic reaction) after administration of Tiotropium. If any of these occur, please consult your doctor immediately
In addition, in common with all inhaled medicines, some patients may experience an unexpected tightness of the chest, coughing, wheezing or breathlessness immediately after inhalation (bronchospasm).
Reporting of side effects:
If you get any side effects, talk to your doctor or pharmacist.This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme (Tel: Freephone 0808 100 3352 or Website: www.mhra.aov.uk/vellowcard1. By reporting side effects you can help provide more information on the safety of this medicine.
Q How to store Tiotropium
Keep out of the sight and reach of children.
Do not use Tiotropium after the expiry date which is stated on the carton and on the inhaler label. The expiry date refers to the last day of the month. Tiotropium inhaler should be discarded at the latest 3 months after first use (see Instructions for Use overleaf).
Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
If your medicine becomes discoloured or show any other signs of deterioration, ask your pharmacist who will advise you what to do.
O Contents of the pack and other information What Tiotropium contains:
The active substance is tiotropium. The delivered dose is 2.5 micrograms tiotropium (as bromide monohydrate) per puff (2 puffs comprise one medicinal dose).
The other ingredients are: Benzalkonium chloride, disodium edetate, purified water, and hydrochloric acid 3.6% for pH adjustment.
What Tiotropium looks like and contents of the pack
The Cardboard carton contains one inhaler and one aluminium cylindrical cartridge containing a clear colourless solution for inhalation. A plastic cap is present on both ends of the inhaler: a green cap covering the mouth piece; and a transparent cap where the cartridge is to be inserted. The inhaler has an indicator on the side showing the number of doses left.
Single pack: 1 Respimat inhaler and 1 cartridge, providing 60 puffs (30 medicinal doses)
Manufacturer and Licence Holder
This medicine is manufactured by Boehringer Ingelheim Pharma GmbH &
Co. KG, Binger Strasse 173, D-55216 Ingelhein am Rhein, Germany and is procured from within the EU. Product Licence Holder: Lexon UK Limited, Unit 18 Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 ORE.
If you have any questions or are not sure about anything, ask your doctor or pharmacist. They will have additional information about this medicine and will be able to advise you.
PL 15184/1619 - Tiotropium 250 microgram, Inhalation solution
Leaflet revision date: 04/04/16
Blind or partially sighted? Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
Patient Information Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Your medicine is called Tiotropium 2.5 meg, inhalation solution and will be referred to as Tiotropium throughout the rest of this leaflet..
What is in this leaflet
Q What Tiotropium is and what it is used for Q What you need to know before you take Tiotropium Q How to take Tiotropium Q Possible side effects Q How to store Tiotropium Q Contents of the pack and other information
Q What Tiotropium is and what it is used for
Tiotropium helps people who have chronic obstructive pulmonary disease (COPD) or asthma to breathe more easily. COPD is a long-term lung disease that causes shortness of breath and coughing. The term COPD is associated with the conditions chronic bronchitis and emphysema. Asthma is a long-term disease characterised by airway inflammation and narrowing of the airways. As COPD and asthma are long-term diseases you should take Tiotropium every day and not only when you have breathing problems or other symptoms. When used to treat asthma you should use Tiotropium in addition to so-called inhaled corticosteroids and long-acting IJ2 agonists.
Tiotropium is a long-acting bronchodilator that helps to open your airways and makes it easier to get air in and out of the lungs. Regular use of Tiotropium can also help you when you have on-going shortness of breath related to your disease, and will help to minimise the effects of the disease on your everyday life. Daily use of Tiotropium will also help to prevent any sudden, short-term worsening of your COPD symptoms which may last for several days.
For correct dosing of Tiotropium please see section 3. How to take Tiotropium and the instructions for use provided on the other side of the leaflet.
Q What you need to know before you take Tiotropium
Please read the following questions carefully. If you can answer any of these questions with ‘Yes’ please discuss this with your doctor before taking Tiotropium
• are you allergic (hypersensitive) to tiotropium, atropine or similar drugs such as ipratropium or oxitropium?
• are you taking any other medicinal products containing ipratropium or oxitropium?
• are you pregnant, do you think you are pregnant, or are you breast-feeding?
• are you suffering from blurred vision, eye pain and/or red eyes, prostate problems or have difficulty passing urine?
• do you have any kidney problems?
• have you suffered from a myocardial infarction during the last 6 months or from any unstable or life threatening irregular heart beat or severe heart failure within the past year?
Do not use Tiotropium
• if you are allergic (hypersensitive) to tiotropium, its active ingredient or any of the other ingredients of this medicine (listed in section 6)
• if you are allergic (hypersensitive) to atropine or substances related to it, e.g. ipratropium or oxitropium
Warnings and precautions
Talk to your doctor before taking Tiotropium.
When taking Tiotropium take care not to let any spray enter your eyes. This may result in eye pain or discomfort, blurred vision, seeing halos around lights or coloured images in association with red eyes (i.e. narrow angle glaucoma). Eye symptoms may be accompanied by headache, nausea or vomiting. Wash your eyes in warm water, stop using tiotropium bromide and immediately consult your doctor for further advice.
If your breathing has got worse or if you experience rash, swelling or itching directly after using your inhaler, stop using it and tell your doctor immediately.
Dry mouth which has been observed with anti-cholinergic treatment may in the long term be associated with dental caries. Therefore, please remember to pay attention to oral hygiene.
Tiotropium is indicated for the maintenance treatment of your chronic obstructive pulmonary disease or asthma. Do not use this medicine to treat a sudden attack of breathlessness or wheezing. Your doctor should have given you another inhaler (“rescue medication”) for this.
Please follow the instructions your doctor has given you.
If you have been prescribed Tiotropium for your asthma it should be added on to inhaled corticosteroids and long-acting IJ2 agonists. Continue taking the inhaled corticosteroids as prescribed by your doctor, even if you feel better.
In case you have suffered from a myocardial infarction during the last 6 months or from any unstable or life threatening irregular heart beat or severe heart failure within the past year, please, inform your doctor. This is important to decide if Spiriva is the right medicine for you to take.
Do not take Tiotropium more frequently than once daily.
You should also contact your doctor if you feel that your breathing is worsening.
If you have cystic fibrosis, tell your doctor because Tiotropium could make your cystic fibrosis symptoms worse.
Children and adolescents
Tiotropium is not recommended for children and adolescents under 18 years. Other medicines and Tiotropium
Please tell your doctor or pharmacist if you are taking, or have recently taken, any other medicines, including medicines obtained without a prescription.
In particular, please tell your doctor or pharmacist if you are taking/have taken anticholinergic drugs, e.g. ipratropium or oxitropium.
No interaction side effects have been reported when Tiotropium has been taken with other products used to treat COPD such as reliever inhalers (e.g. salbutamol), methylxanthines (e.g. theophylline), antihistamines, mucolytics (e.g. ambroxol), leukotriene modifiers (e.g. montelukast), cromones, anti-lgE treatment (e.g. omalizumab) and/or inhaled or oral steroids (e.g. budesonide, prednisolone).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. You should not use this medicine unless specifically recommended by your doctor.
Driving and using machines
No studies on the effects and the ability to drive and use machines have been performed. In case dizziness or blurred vision occurs the ability to drive and use machinery may be influenced.
Q How to take Tiotropium
Always take this medicine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Tiotropium is for inhalation use only.
The recommended dose for adults is:
Tiotropium is effective for 24 hours so you will need to use Tiotropium only ONCE A DAY, if possible at the same time of the day. Each time you use it take TWO PUFFS.
As COPD and asthma are long-term diseases take Tiotropium every day and not only when you experience breathing problems. Do not take more than the recommended dose.
Tiotropium is not recommended for use in children and adolescents below 18 years due to lack of data on safety and efficacy.
Make sure that you know how to use your Tiotropium inhaler properly.
The instructions for use of the Tiotropium inhaler are provided on the other side of this leaflet.
If you take more Tiotropium than you should
If you take more Tiotropium than two puffs in one day talk to your doctor immediately. You may be at a higher risk of experiencing a side effect such as dry mouth, constipation, difficulties passing urine, increased heart beat or blurred vision.
If you forget to take Tiotropium
If you forget to take your daily dose (TWO PUFFS ONCE A DAY), don’t worry. Take it as soon as you remember but do not take two doses at the same time or on the same day. Then take your next dose as usual.
If you stop taking Tiotropium
Before you stop taking Tiotropium, you should talk to your doctor or your pharmacist. If you stop taking Tiotropium the signs and symptoms of COPD may worsen.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
nstructions For Use
Prepare for first use
Introduction
Remove clear base
CAP
CLEAR BASE


Tiotropium (tiotropium bromide). Read these Instructions for Use before you start using Tiotropium.
You will need to use this inhaler only ONCE A DAY. Each time you use it take TWO PUFFS.
MOUTHPIECE-
AIR VENT-
DOSE-RELEASE BUTTON
SAFETY CATCH
PIERCING ELEMENT
CARTRIDGE
• If Tiotropium has not been used for more than 7 days release one puff towards the ground.
• If Tiotropium has not been used for more than 21 days repeat steps
4 to 6 under ‘Prepare for first Use’ until a cloud is visible. Then repeat steps 4 to 6 three more times.
• Do not touch the piercing element inside the clear base.
1- • Your Tiotropium inhaler contains 60 puffs (30 doses) if used as indicated (two puffs/once daily).
• The dose indicator shows approximately how much medication is left.
SAFETY CATCH
CLEAR BASE

Insert cartridge
2. • Insert the narrow end of the cartridge into the inhaler.
• Place the inhaler on a firm surface and push down firmly until it clicks into place.

Replace clear base 3. • Put the clear base back into place until it clicks.
How to care for your Tiotropium
CLEAR BASE
Clean the mouthpiece including the metal part inside the mouthpiece with a damp cloth or tissue only, at least once a week.
Any minor discoloration in the mouthpiece does not affect your Tiotropium inhaler performance. If necessary, wipe the outside of your Tiotropium inhaler with a damp cloth.
When to get a new Spiriva Respimat
Turn
4. • Keep the cap closed.
DOSE
INDICATOR

EMPTY
FULL
ET]
• Turn the clear base in the direction of the arrows on the label until it clicks (half a turn).
ARROWS

Open
• Your Tiotropium inhaler contains 60 puffs (30 doses) if used as indicated (two puffs/once daily).
• The dose indicator shows approximately how much medication is left.
• When the dose indicator enters the red area of the scale you need to get a new prescription; there is approximately medication for 7 days left
(14 puffs).
• Once the dose indicator reaches the end of the red scale, your Spiriva Respimat locks automatically - no more doses can be released.
At this point, the clear base cannot be turned any further.
• Tiotropium should be discarded three months after you have prepared it for first use, even if it has not been fully used or used at all.
5. • Open the cap until it snaps fully open.
CAP

Press 6- • Point the inhaler toward the ground.
• Press the dose-release button
• Close the cap
• Repeat steps 4-6 until a cloud is visible.
• After a cloud is visible, repeat steps 4-6 three more times.
Your inhaler is now ready to use. These steps will not affect the number of doses available.After preparation your inhaler will be able todeliver 60 puffs (30 doses).
DOSE
RELEASE
BUTTON
Daily use
TURN
• Keep the cap closed.
• TURN the clear base in the direction of the arrows on the label until it clicks (half a turn).

OPEN
• OPEN the cap until it snaps fully open.
CAP

PRESS
• Breathe out slowly and fully.
• Close your lips around the mouthpiece without covering the air vents. Point your Inhaler to the back of your throat.
• While taking a slow, deep breath through your mouth, PRESS the dose-release button and continue to breathe in slowly for as long as comfortable.
• Hold your breath for 10 seconds or for as long as comfortable.
• Repeat TURN, OPEN, PRESS for a total of 2 puffs.
• Close the cap until you use your inhaler again.
Answers to Common Questions
It is difficult to insert the cartridge deep enough.
Did you accidentally turn the clear base before inserting the cartridge?
Open the cap, press the dose-release button, then insert the cartridge.
Did you insert the cartridge with the wide end first?
Insert the cartridge with the narrow end first.
The dose indicator on the Tiotropium reaches zero too soon.
Did you use Tiotropium as indicated (two puffs/Once daily)? Tiotropium will last 30 days if used at two puffs once daily.
Did you turn the clear base before you inserted the cartridge?
The dose indicator counts each turn of the clear base regardless whether a cartridge has been inserted or not.
Did you spray in the air often to check whether the Tiotropium is working?
Once you have prepared Tiotropium, no test-spraying is required if used daily.
Did you insert the cartridge into a used Tiotropium?
Always insert a new cartridge into a NEW Tiotropium.
I cannot press the dose-release button.
Did you turn the clear base?
If not, turn the clear base in a continuous movement until it clicks (half a turn).
Is the dose indicator on the Tiotropium pointing to zero?
The Tiotropium inhaler is locked after 60 puffs (30 medicinal doses). Prepare and use your new Tiotropium inhaler.
My Tiotropium sprays automatically.
Was the cap open when you turned the clear base?
Close the cap, then turn the clear base.
Did you press the dose-release button when turning the clear base?
Close the cap, so the dose-release button is covered, then turn the clear base.
Did you stop when turning the clear base before it clicked?
Turn the clear base in a continuous movement until it clicks (half a turn).
I cannot turn the clear base.
Did you turn the clear base already?
If the clear base has already been turned, follow steps “OPEN” and “PRESS” under “Daily Use” to get your medicine.
Is the dose indicator on the Tiotropium pointing to zero?
The Tiotropium inhaler is locked after 60 puffs (30 medicinal doses). Prepare and use your new Tiotropium inhaler.
My Tiotropium doesn’t spray.
Did you insert a cartridge?
If not, insert a cartridge.
Did you repeat TURN, OPEN, PRESS less than three times after inserting the cartridge?
Repeat TURN, OPEN, PRESS three times after inserting the cartridge as shown in the steps 4 to 6 under “Prepare for first Use”.
Is the dose indicator on the Tiotropium pointing to 0?
If the dose indicator points to 0, you have used up all your medication and the inhaler is locked.
Once your Tiotropium is assembled, do not remove the clear base or the cartridge. Always insert a new cartridge into a NEW Tiotropium.
POM
15184/0131
Other useful information
• Bathing, swimming, showering or exercising should not affect the patch if it has been correctly applied. You may wear the patch under your swimming costume.
• If the patch does come off in the bath or shower it may be reapplied.
Shake it to remove any water, dry the skin thoroughly, allow the area to cool and put it on again in the usual way.
• Never apply a patch on a sweaty area or after a hot bath or shower. Wait until the skin is completely cool and dry.
• Sunbathing: always make sure your patch is covered by clothing.
• Using a sunbed: cover up the patch
• The drug in your patch is a gel which is colourless. This does not mean that the patch does not contain any medication.
How long to use Estracombi TTS
Estracombi TTS should be used only as long as needed, possibly for several months or more. This will help to control your symptoms.
While you are using Estracombi TTS you should go to the doctor regularly to discuss the possible risks and benefits associated with HRT, and whether you still need the treatment. Your doctor will aim to give you the lowest possible dose for the shortest possible length of time to treat your symptoms.
If you forget to use Estracombi TTS
If you forget to apply a patch, apply a new patch as soon as you remember. No matter what day that happens, go back to changing this patch on the same day as you usually do. There is an increased chance of breakthrough bleeding or spotting if there is a break in treatment.
Q Possible side effects
Estracombi TTS is suitable for most people, but, like all medicines, there are sometimes side effects.
Stop using Estracombi TTS immediately and tell your doctor if you develop any of the following:
• Signs of an allergic reaction (difficulty in breathing, tight chest, itching all over, generalised swelling or itching)
• Signs of a blood clot (swollen legs, breathlessness, chest pain)
• Migraine or unusually severe headaches, or other signs of a stroke
• You become pregnant
• Signs of jaundice (yellowing of your skin or eyes).
The side effects listed below have also been reported:
More than 10 % of people have experienced:
• Headache
• Redness and itching where the patch has been applied
• Tender or painful breasts
• Painful periods and other menstrual problems.
Up to 1 in 10 people have experienced:
Dizziness, depression, nervousness, sleeplessness, mood changes Feeling sick, stomach ache, indigestion, wind, diarrhoea Acne, rash, dry skin and itching
A condition called endometrial hyperplasia (thickening of the lining of the womb)
Enlarged breasts, cramps
Heavy periods, vaginal discharge, bleeding or irritation Pain, back pain, restlessness Abnormal weight gain
Fluid retention (swelling or accumulation of fluid in the lower legs or ankles).
Up to 1 in 100 people have experienced:
Migraine
Breast cancer
Rise in blood pressure
Being sick or feeling bloated,
Abnormal liver function test results
Peeling and blistered skin where the patch is applied
Changes in the pigmentation in your skin (lightening or darkening of your skin colour)
Up to 1 in 1,000 people have experienced:
Blood clots in the veins
Gallstones, other disease of the gall bladder, jaundice
Fibroids, cysts or polyps of the ovarian, uterine or cervical regions which
might cause pain or bleeding
Allergic reactions
Changes in libido
Tingling of the hands and feet.
Other side effects include:
Estrogen-dependent conditions such as blood clots, worsening varicose veins, tumours including breast cancer, cancer of the endometrium, heart attack, stroke, dementia (these are discussed in Section 2) and gall bladder disease.
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using Estracombi TTS. Ask your doctor or pharmacist to answer any questions you may have.
Q How to store the patches
• KEEP THIS MEDICINE OUT OF THE REACH AND SIGHT OF CHILDREN
• Do not store above 25°C.
• Store in the original packaging. Do not refrigerate.
Do not use this medicine after the expiry date shown on the carton label. If your doctor tells you to stop using the patches, take them back to the pharmacist for safe disposal. Only keep the patches if your doctor tells you to do so.
If the patches become brittle, discoloured or show any signs of deterioration, ask your pharmacist who will advise you what to do.
Q Further information Your medicine
Your medicine is called Estracombi TTS patches. They are available as a calendar pack which contains 4 patches, marked (A) and 4 patches marked (B). The patches are individually sealed in foil pouches in strips of 2 pouches. The name and strength are printed on the labels.
What is in your medicine
Each patch marked (A) contains 4mg of the active ingredient, estradiol. While you are wearing an Estracombi TTS patch (marked A) your body will absorb approximately 50 micrograms of estradiol per day.
Each patch marked (B) contains 10mg of the active ingredient estradiol, and 30mg of the active ingredient norethisterone acetate. While you are wearing an Estracombi TTS patch (marked B) your body will absorb approximately 50 micrograms of estradiol per day and 250 micrograms of norethisterone acetate per day.
Estracombi TTS patches also contain the following inactive ingredients: Ethanol, hydroxypropylcellulose, light liquid paraffin, polyethylene terephthalate, ethylene vinyl acetate co-polymer and poly-isobutylene.
Manufacturer and Licence Holder
The patches are manufactured by Novarits Pharma, S.A. Basle, Switzerland, and are procured from within the EU and repackaged by the Product Licence Holder: Lexon (UK) Limited, Unit 18, Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 ORE.
PL Number:
Estracombi TTS is a registered trademark of Novartis AG.
Leaflet revision date: 21/01/09
Blind or partially sighted?
Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
Estracombi® TTS Patches
Ref:0131/210109/1/F
(estradiol & norethisterone acetate)
Patient Information Leaflet
Your medicine is called Estracombi TTS Patches, and will be referred to as Estracombi TTS throughout this leaflet.
What you need to know about Estracombi TTS
Your doctor has decided that you need this medicine to help treat your condition.
Please read this leaflet carefully before you start to use the patches. It contains important information. Keep the leaflet in a safe place because you may want to read it again.
If you have any other questions, or if there is something you don’t understand, please ask your doctor or pharmacist.
This medicine has been prescribed for you. Never give it to someone else. It may not be the right medicine for them even if their symptoms seem to be the same as yours.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
Q What Estracombi TTS is, and what it’s used for Q Things to consider before you start to use Estracombi TTS Q How to use Estracombi TTS Q Possible side effects Q How to store the patches Q Further information
Q What Estracombi TTS is and what it’s used for
Estracombi TTS is known as a combined HRT product. The pack contains two different patches which you stick on your skin, Estracombi TTS (A) and Estracombi TTS (B). Estracombi TTS (A) is a round patch which contains a supply of estradiol. Estracombi TTS (B) is a goggle-shaped patch which contains two active ingredients, estradiol and norethisterone acetate.

Estracombi TTS (A) Estracombi TTS (B)
The active ingredients are released from the patch and absorbed through the skin into your blood stream. This is why the patches are called transdermal patches.
Estradiol is one of a group of hormones called estrogens which are the natural female sex hormone produced in large amounts by the ovaries before the menopause. After the menopause the ovaries produce less estrogen. Norethisterone acetate is a different sort of hormone called a progestogen. It is similar to another female hormone, progesterone. It helps to protect the lining of the womb in women who have not had a hysterectomy (see the section below on endometrial cancer).
Estracombi TTS is used as hormone replacement therapy (HRT) to relieve the symptoms of the menopause. The menopause can occur naturally or as the result of surgery.
Estracombi TTS can also be used to prevent osteoporosis (thinning of the bones), when you have a high risk of future fractures, and if you are unable to take other medicines for this purpose.
• To relieve the symptoms of the menopause:
Estracombi TTS is used to help relieve the uncomfortable symptoms which you sometimes get during and after the menopause (the time when menstrual periods stop). Menopause occurs naturally in all women, usually between the ages of 45 and 55. It also occurs in younger women who have had their ovaries removed by surgery. The reduced levels of estrogen can cause unpleasant symptoms such as hot face, neck and chest, hot flushes (sudden waves of heat and sweating all over), sleep problems, irritability and depression. Some women also have problems with urine control, or with a dry vagina which may cause discomfort during or after sexual intercourse. Taking estrogens can reduce or eliminate these symptoms.
• To prevent osteoporosis:
After the age of 40, and especially after the menopause, some women are at risk of developing osteoporosis. This is when the bones become thinner and weaker and more likely to break, especially the bones of the spine, hip and
wrist. Lack of estrogens increases the risk of osteoporosis. Taking estrogens after the menopause slows down bone loss and can help to prevent it. The protective effects on the bone cease once treatment is stopped. You should discuss the benefits and risks of Estracombi TTS and other therapies with your doctor.
Q Things to consider before you start to use Estracombi TTS
Read this section carefully because, there are some conditions your doctor should know about before you start your treatment.
Medical check-ups
Before you start using HRT, your doctor should ask you about your own, and your family’s, medical history. Your doctor may decide to examine your breasts and/or your abdomen and may do an internal examination - but only if these examinations are necessary for you, or you have any special concerns. Once you have started HRT, you should see your doctor for regular check-ups (at least once a year). At these check-ups your doctor may discuss with you the benefits and risks of continuing with HRT.
While you are using HRT make sure that you:
• Go for regular breast screening and cervical smear tests.
• Regularly check vour breasts for any changes such as dimnlina of the skin, changes in the nipple, a discharge from the nipple, or any lumps you can see or feel.
Some people MUST NOT use Estracombi TTS. Talk to your doctor if:
• you have ever had any unusual or allergic reaction to estrogens, progestagens or to any other components of the patch, (These are listed at the end of the leaflet.)
• you have, or have ever had, breast cancer, (See the section below on breast cancer.)
• you have, or have ever had, cancer of the endometrium (lining of the womb) or any other cancer which is sensitive to estrogens, (See the sections below on endometrial and ovarian cancer.)
• you have a blood disease called porphyria,
• you have, or have ever had, a blood clot in a vein in your leg or anywhere else (a ‘deep vein thrombosis’) or a clot that has travelled to your lung or another part of your body (an ‘embolus’), (See the section below on blood clots.)
• you have ever had a heart attack, stroke or angina, (See the sections below on heart disease and stroke.)
• you have had any unexpected bleeding or very heavy bleeding from the vagina,
• you have a history of severe liver, kidney or heart disease,
• you have the genetic disorders Dubin-Johnson syndrome or Rotor syndrome which affect the blood and cause hyperbilirubinaemia,
• you are pregnant or breastfeeding.
Estracombi TTS should not be used in children.
You should also ask yourself these questions before using the patch. If the answer to any of these questions is YES, tell your doctor or pharmacist because Estracombi TTS might not be the right medicine for you.
• Has anyone in your immediate family had breast cancer?
• Do you have fibroids or any other growths in your womb?
• Do you have endometriosis (a condition which causes painful periods)?
• Do you have high blood pressure?
• Do you have any problems with your liver including jaundice or a widespread rash?
• Do you have diabetes, epilepsy or asthma?
• Do you get migraine or other bad headaches?
• Do you have a condition called lupus (SLE)?
• Do you have problems with your hearing?
• Do you have any of the conditions which put you at increased risk of blood clots (see below)?
• Do you have a high level of cholesterol or other fats in your blood?
• Do you have heart or kidney problems?
• Have you ever had problems with your gall bladder, such as gallstones?
• Are you over 65?
Are you taking other medicines?
Some medicines can interfere with your treatment. Tell your doctor or pharmacist if you are taking any of the following:
• medicines to treat epilepsy such as phenobarbital, phenytoin and
carbamazepine, _ .
• antibiotics and other medicines to treat infections (e.g. rifampicin, rifabutin, nevirapine, efavirenz, ritonavir, nelfinavir),
• the herbal medicine St John’s Wort (also known as Hypericum perforatum).
Always tell your doctor or pharmacist about all the medicines you are taking. This means medicines you have bought yourself as well as medicines on prescription from your doctor.
Before you have a blood test remind your doctor that you are using Estracombi TTS as it may affect the result.
Will there be any problems with driving or using machinery?
No problems have been reported.
Using contraception whilst taking Estracombi TTS
Estracombi TTS is not a contraceptive, nor a fertility treatment.
If you are using an oral or other hormone contraceptive, e.g. the pill or depot injection, you must change to a non-hormone contraceptive, for example, a diaphragm or condom, BEFORE starting Estracombi TTS.
If you have been told that you don’t need to use a contraceptive any longer, you won’t need one while you are using Estracombi TTS even if you have a monthly bleed.
Other special warnings
HRT will not help to prevent heart disease.
As well as benefits, HRT has some risks which you need to consider when you are deciding whether to use it, or whether to carry on using it. These are:
Effects on vour heart or circulation
i. Heart disease
HRT is not recommended for women who have heart disease, or have had heart disease recently. If you have ever had heart disease, talk to your doctor to see if you should be using HRT.
Studies with one type of HRT (containing conjugated estrogen plus a progestogen) have shown that women may be slightly more likely to get heart disease during the first year of taking the medication. For other types of HRT, the risk is likely to be similar, although this is not yet certain.
If you get:
A pain in your chest that spreads to your arm or neck,
See a doctor as soon as possible and do not use any more HRT until your doctor says you can. This pain could be a sign of heart disease.
ii. Stroke
Recent research suggests that HRT slightly increases the risk of having a stroke. Other things that can increase the risk of stroke include:
• Getting older
• High blood pressure
• Smoking
• Drinking too much alcohol
• An irregular heartbeat
If you are worried about any of these things, or if you have had a stroke in the past, talk to your doctor to see if you ought to be using HRT.
If you get:
• Unexplained migraine-type headaches, with or without disturbed vision, See a doctor as soon as possible and do not use any more HRT until your doctor says you can. These headaches may be an early warning sign of a stroke.
iii. Blood clots
Using HRT may increase the risk of blood clots in the veins (also called deep vein thrombosis, or DVT), especially during the first year. These blood clots are not always serious, but if one travels to the lungs, it can cause chest pain, breathlessness, collapse or even death. This condition is called pulmonary embolism, or PE.
DVT and PE are examples of a condition called venous thromboembolism, or VTE.
You are more likely to get a blood clot if:
• You are seriously overweight
• You have had a blood clot before
• Any of your close family have had blood clots
• You have had one or more miscarriages
• You have any blood clotting problem that needs treatment with a medicine such as warfarin
• You’re off your feet for a long time because of major surgery, injury or illness
• You are going on a long journey and will be immobile for some time
• You have a rare condition called systemic lupus erythematosus (SLE - a connective tissue disease).
If any of these things apply to you, talk to your doctor to see if you should be using HRT.
If vnu get:
• Painful swelling in your leg
• Sudden chest pain
• Difficulty breathing
See a doctor as soon as possible and do not use any more HRT until your doctor says you can. These may be signs of a blood clot.
If you’re going to have suraerv. make sure your doctor knows about it. You may need to stop using HRT about 4 to 6 weeks before the operation, to reduce the risk of a blood clot. Your doctor will tell you when you can start using HRT again.
A comparison - the approximate risk of stroke or blood clots in women not using, or using, HRT over a 5 year period.
|
Women in their 50s |
Women in their 60s | |||
|
Not using HRT |
Using HRT |
Not using HRT |
Using HRT | |
|
Stroke |
3 in 1,000 |
4 in 1,000 |
11 in 1,000 |
15 in 1,000 |
|
Blood clot |
3 in 1,000 |
7 in 1,000 |
8 in 1,000 |
17 in 1,000 |
Effects on vour risk of developing cancer i. Breast cancer
Women who have breast cancer, or have had breast cancer in the past, should not use HRT.
Using HRT slightly increases the risk of breast cancer; so does having a late menopause. The risk for a post-menopausal woman using estrogen-only HRT for 5 years is about the same as for a woman of the same age who’s still having periods over that time and not using HRT. The risk for a woman who is using combined HRT (estrogen plus progestogen) is higher than for estrogen-only HRT (but combined HRT is beneficial for the endometrium, see Endometrial cancer, below).
For all kinds of HRT, the extra risk of breast cancer goes up the longer you use it, but returns to normal within about 5 years of stopping HRT.
Your risk of breast cancer is also higher if:
• You have a close relative (mother, sister or grandmother) who has had breast cancer
• You are seriously overweight.
If you notice any changes in your breast, such as:
• Dimpling of the skin
• Changes in the nipple or a discharge from the nipple
• Any lumps you can see or feel
Make an appointment to see your doctor as soon as possible.
A comparison - the risk of developing breast cancer: the number of women aged 50 who will get breast cancer by the time they are 65.
|
Not using HRT |
Using estrogen-only HRT |
Using combined HRT | ||
|
For 5 years |
For 10 years |
For 5 years |
For 10 years | |
|
32 in 1,000 |
33.5 in 1,000 (1 -2 cases extra) |
37 in 1,000 (5 cases extra) |
38 in 1,000 (6 cases extra) |
51 in 1,000 (19 cases extra) |
ii. Endometrial cancer (cancer of the lining of the womb)
Estracombi TTS is a combined HRT product containing an estrogen and a progestogen.
Using estrogen-only HRT for a long time can increase the risk of cancer of the lining of the womb. Taking a progestogen as well as the estrogen helps to lower the extra risk.
If you still have vour womb, your doctor may prescribe a progestogen as well as estrogen. They can be prescribed separately, or as a combined HRT product.
If you have had vour womb removed (a hysterectomy), your doctor will discuss with you whether you can safely take estrogen without a progestogen.
If you’ve had vour womb removed because of endometriosis, any endometrium left in your body may be at risk. So your doctor may prescribe HRT that includes a progestogen as well as an estrogen.
Week
Patches
|
1 |
2 |
3 |
4 |
|
1 2 |
3 4 |
1 2 |
3 4 |
Estracombi TTS (A) Estracombi TTS (B)


A comparison - the risk of developing endometrial cancer
Women who still have their uterus and who do not use HRT
About 5 in 1,000 women aged 50 will get endometrial cancer by the time they are 65.
Women who have used estrogen-only HRT
The number will be 2-12 times higher depending on the dose and length of use.
Women who are using estrogen plus progestogen HRT
The risk of endometrial cancer is substantially reduced.
If you get breakthrough bleeding or spotting, it’s usually nothing to worry about, especially during the first few months of taking HRT.
But if the bleeding or spotting:
• Carries on for more than the first few months
• Starts after you’ve been on HRT for a while
• Carries on even after you’ve stopped using HRT
Make an appointment to see your doctor. It could be a sign that your endometrium has become thicker.
Hi. Ovarian cancer (cancer of the ovaries)
Cancer of the ovaries is very rare, but it is serious. It can be difficult to diagnose, because there are often no obvious signs of the disease.
Some studies have indicated that using estrogen-only HRT for more than 5 years may increase the risk of ovarian cancer. It is not yet known whether other kinds of HRT increase the risk in the same way.
Effects on vour risk of developing dementia
HRT will not prevent memory loss. In one study of women who started using combined HRT after the age of 65, a small increase in risk of dementia was observed.
Q How to use Estracombi TTS patches
The doctor will tell you how to use Estracombi TTS. Always follow his/her instructions carefully. The information will be on the pharmacist’s label. Check the label carefully. If you are not sure, ask your doctor or pharmacist. Do not change or stop the treatment without talking to your doctor.
Starting to use the patches
Estracombi TTS patches are applied to the skin. You wear a patch all the time. For one treatment cycle you need 4 patches of Estracombi TTS (A) and 4 patches of Estracombi TTS (B).
If you are still having fairly regular periods, start using Estracombi TTS no more than 5 days after the beginning of your period. If your periods have stopped, you can start using Estracombi TTS at any convenient time.
If you have been using a continuous combined HRT product (where estrogen and progestogen are given every day without interruption) you can start Estracombi TTS on any convenient day.
If you are changing from a sequential HRT treatment (where the progestogen is added for 12-14 days of the cycle) you should finish your current cycle of treatment before starting Estracombi TTS. Menstrual type bleeding often occurs at the end of sequential HRT treatment cycles, try to start Estracombi TTS on the first day of bleeding.
It is very important that you use all the Estracombi TTS (A) patches followed by all the Estracombi TTS (B) patches in the correct order each month. If for any reason a patch becomes unusable or you lose it, apply a fresh patch of the same type, preferably taken from a spare pack. You should then keep this pack as a spare until its expiry date.
Consult your doctor if this is not possible or leaves you short of patches.
The treatment cycle starts with an Estracombi TTS (A) patch.
During weeks 1 and 2 you apply the Estracombi TTS (A) patches, changing the patch every 3 to 4 days. During weeks 3 and 4 you use the Estracombi TTS (B) (0.25/50) patches, also changing the patch every 3 to 4 days.
The progestogen, norethisterone acetate, causes a monthly bleed in most women. This bleeding usually starts at the end of the Estracombi TTS (B) treatment phase. The duration and the amount of bleeding are usually similar to that of a normal period. Tell your doctor if you have heavy or irregular bleeding after a few months of treatment.
You should apply the first Estracombi TTS (A) patch of the next cycle immediately, even if the bleeding has not stopped.
About the patch
Where to apply the patch
Stick the patch on to a hairless area of skin below the waist. Most women find that the buttock is the best place. Choose an area of the buttock where the skin is not inflamed, broken, or irritated. You could also try the lower back, hip or abdomen. Avoid the waistline since the patch might get rubbed off.
Never put a patch on or near the breasts.
Choose a clean, dry area of skin. To help the patch stick, the skin should be clean, dry, and free of creams, lotions, oil, or powder. You should use a different area of skin each time. Wait a week before using the same area again. Avoid skin which is red or irritated.
Do not expose the patch to direct sunlight.
Opening the sachets
Each patch is sealed in an airtight sachet. A double-pack for one week's treatment consists of two sachets joined by a perforated seam. Tear open one of the sachets at the notch (do not use scissors) and take out the patch. Make sure that the second sachet is undamaged, because the patch becomes ineffective when exposed to the air before use. If you happen to tear open both sachets at the same time, throw one of them away.

Removing the lining
A stiff, transparent protective lining covers the sticky side of the patch, i.e. the side that will be placed against your skin. Loosen this lining by rubbing the edge of the patch between your thumb and forefinger. Holding the patch at the edge, peel off the protective lining and throw it away. Try to avoid touching the adhesive. Now apply the patch.

Applying the patch
Press the sticky side of the patch firmly onto the spot you have chosen with the palm of your hand. Hold it there for about 10-20 seconds. Make sure that it sticks well, especially around the edges. Do not test the patch by pulling it once it is on your skin.
When and how to remove the patch
The patch should be changed twice each week i.e. every 3-4 days, always on the same two days, e.g. Mondays and Thursdays. You may find it helpful to tick the box on the packaging for the day of the week when you apply your first Estracombi TTS patch. This then shows the two days each week when you should change your patch. When you finish an Estracombi TTS pack, start the next pack straightaway. Do not have a break between packs.
When the time comes to change the patch, peel it off and fold it in half with the sticky side inside. Dispose of the patch carefully with normal household waste, making sure that it is out of the reach of children because it will still contain some medication. Stick a new patch onto a different area of skin.
What to do if a patch comes off
If a patch falls off the same patch may be put on a different area (see Where to apply the patch). Make sure you choose a clean, dry, lotion-free area of the skin. No matter what day this happens, go back to changing the patch on the same days as usual.
When you finish an Estracombi TTS pack, i.e. after one cycle, start the next pack straight away. Do not have a break between packs.
Ref:0131/210109/1/B Page 3