Valcyte 50 Mg/Ml Powder For Oral Solution

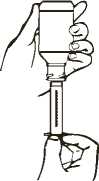
DISPENSER
child
safety
bottle
cap

&UOOS-Biuoor-Bmooe— Biuhe— Bui ooi —
PACKAGE LEAFLET:
INFORMATION FOR THE USER_
Valcyte® <@>
50 mg/ml
powder for oral solution
valganciclovir
Read all of this leaflet carefully before you
start taking this medicine because it
contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Valcyte is and what it is used for
2. What you need to know before you take Valcyte
3. How to take Valcyte
4. Possible side effects
5. How to store Valcyte
6. Contents of the pack and other information
1. What Valcyte is and what it is used for
Valcyte belongs to a group of medicines, which work directly to prevent the growth of viruses. In the body the active ingredient in the powder, valganciclovir, is changed into ganciclovir. Ganciclovir prevents a virus called cytomegalovirus (CMV) from multiplying and invading healthy cells. In patients with a weakened immune system, CMV can cause an infection in the body’s organs. This can be life threatening.
Valcyte is used:
• for the treatment of CMV-infections of the retina of the eye in adult patients with acquired immunodeficiency syndrome (AIDS). CMV-infection of the retina of the eye can cause vision problems and even blindness.
• to prevent CMV-infections in adults and children who are not infected with CMV and who have received an organ transplant from somebody who was infected by CMV.
2. What you need to know before you take Valcyte
Do not take Valcyte:
• if you are allergic to valganciclovir or any of the other ingredients of this medicine (listed in section 6).
• if you are allergic to ganciclovir, acyclovir or valaciclovir, which are medicines used to treat other virus infections.
• if you are breast-feeding.
Warnings and precautions
Talk to your doctor or pharmacist before taking Valcyte.
Take special care with Valcyte
• if you have low
numbers of white blood cells, red blood cells or platelets (small cells involved in blood clotting) in your blood. Your doctor will carry out blood tests before you start taking Valcyte and more tests will be done while you are taking the medication.
• if you are having radiotherapy.
• if you have a problem with your kidneys.
Your doctor may need to prescribe a reduced dose for you and may need to check your blood frequently during treatment.
Other medicines and Valcyte
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including medicines obtained without a prescription.
If you take other medicines at the same time as taking Valcyte the combination could affect the amount of drug that gets into your blood stream or could cause harmful effects. Tell your doctor if you are already taking medicines that contain any of the following:
• imipenem-cilastatin (an antibiotic). Taking this with Valcyte can cause convulsions (fits)
• zidovudine, didanosine, lamivudine, tenofovir, abacavir, emtricitabine or similar kinds of drugs used to treat AIDS
• ribavirin, pegylated interferons, adefovir and entecavir used to treat Hepatitis B/C
• probenecid (a medicine against gout).
Taking probenecid and Valcyte at the same time could increase the amount of ganciclovir in your blood
• mycophenolate mofetil (used after transplantations)
• vincristine, vinblastine, adriamycin, hydoxyurea or similar kinds of drugs to treat cancer
• cidofovir or foscarnet used against viral infections
• trimethoprim, trimethoprim/sulpha combinations and dapsone (an antibiotic)
• pentamidine (drug to treat parasite or lung infections)
• flucytosine or amphotericin B (anti-fungal agents)
Valcyte with food and drink
Valcyte should be taken with food. If you are unable to eat for any reason, you should still take your dose of Valcyte as usual.
Pregnancy, breast-feeding and fertility
You should not take Valcyte if you are pregnant unless your doctor recommends it. If you are pregnant or planning to become pregnant you must tell your doctor. Taking Valcyte when you are pregnant could harm your unborn baby.
You must not take Valcyte if you are breastfeeding. If your doctor wants you to begin treatment with Valcyte you must stop breastfeeding before you start taking your medication.
Women of childbearing age must use effective contraception when taking Valcyte.
Men whose partners could become pregnant should use condoms while taking Valcyte and should continue to use condoms for 90 days after treatment has finished.
Driving and using machines
Do not drive or use any tools or machines if you feel dizzy, tired, shaky or confused while taking this medicine.
Ask your doctor or pharmacist for advice before taking any medicine. 1
Valcyte contains sodium
For patients on a sodium-controlled diet, this medicinal product contains a total of 0.188 mg/ml sodium.
3. How to take Valcyte
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
You have to be careful when handling the Valcyte solution. You should avoid getting the solution on your skin or in your eyes. If you accidentally get the solution on your skin, wash the area thoroughly with soap and water. If you accidentally get any solution in your eyes, rinse your eyes thoroughly with water.
You must stick to the daily dose of the oral solution as instructed by your doctor to avoid overdose.
Valcyte oral solution should, whenever possible, be taken with food - see section 2.
It is important that you use the dispenser provided in the pack to measure your dose of Valcyte solution. Two dispensers are provided in case one of them gets lost or damaged. Each dispenser is designed to measure up to a 500 mg amount of solution in 25 mg increments.
Always wash the dispenser thoroughly and allow it to dry after you have taken your dose.
Contact your doctor or pharmacist if both dispensers are lost or damaged, and they will advise you on how to continue to take your medication.
Adults:
Prevention of CMV disease in transplant patients
You should start to take this medicine within 10 days of your transplant. The usual dose is 900 mg Valcyte solution taken ONCE daily. Use the dispenser provided to take two 450 mg amounts (i.e. 2 dispensers filled to 450 mg graduation) of solution. You should continue with this dose for up to 100 days. If you have received a kidney transplant, your doctor may advise you to take the dose for 200 days.
Treatment of active CMV retinitis in AIDS patients (called induction treatment)
The usual dose is 900 mg of Valcyte solution taken TWICE a day for 21 days (three weeks). Use the dispenser provided and take two 450 mg amounts (i.e. 2 dispensers filled to 450 mg graduation) of the solution in the morning and two 450 mg amounts (i.e.
2 dispensers filled to 450 mg graduation) in the evening.
Do not take this dose for more than 21 days unless your doctor tells you to, as this may increase your risk of possible side effects.
Longer term treatment to prevent recurrence of active inflammation in AIDS patients with CMV retinitis (called maintenance treatment)
The usual dose is 900 mg Valcyte solution taken ONCE daily. Use the dispenser provided and take two 450 mg amounts of solution (i.e. 2 dispensers filled to 450 mg graduation).You should try to take the solution at the same time each day. Your doctor will advise you how long you should continue to take Valcyte. If your retinitis worsens while you are on this dose, your doctor may tell you to repeat the induction treatment (as above) or may decide to give you a different medicine to treat the CMV infection.
Elderly patients
Valcyte has not been studied in elderly patients.
Patients with kidney problems
If your kidneys are not working properly, your doctor may instruct you to take a lower dose of Valcyte solution each day. It is very important that you follow the dose prescribed by your doctor.
Patients with liver problems
Valcyte has not been studied in patients with liver problems.
Use in children and adolescents:
Prevention of CMV disease in transplant patients
Children should start to take this medicine within 10 days of their transplant. The dose given will vary depending on the size of the child and should be taken ONCE daily. Your doctor will decide the most appropriate dose based on your child’s height, weight and renal function. You should continue with this dose for up to 100 days. If your child has received a kidney transplant, your doctor may advise you to take the dose for 200 days.
Use the dispenser provided in the pack to measure the dose of Valcyte solution.
Method and route of administration
It is recommended that the Valcyte solution be prepared by the pharmacist prior to it being provided to you.
Once the solution has been prepared, follow the instructions below to withdraw and take your medication.
-mu
plunger
<- tip
1. Shake closed bottle well for about 5 seconds before each use.
2. Remove the child-resistant cap.
3. Before inserting the tip of the dispenser into bottle adapter, push the plunger completely down toward the tip of the dispenser. Insert tip firmly into opening of the bottle adapter.
4. Turn the entire unit (bottle and dispenser) upside down.
5. Pull the plunger out slowly until the desired amount of solution is withdrawn into the dispenser (see diagram).
6. Turn the entire unit right side up and remove the dispenser slowly from the bottle.
7. Dispense directly into mouth and swallow. Do not mix with any liquid prior to dispensing.
Please turn over ^ 10163234 GB
8. Close bottle with child-resistant cap after each use.
9. Immediately after administration: Disassemble the dispenser, rinse under running tap water and air dry prior to next use.
Care should be taken to avoid contact of the skin with the solution. If such contact occurs, wash thoroughly with soap and water.
Do not use the solution after the expiry date which is 49 days from the day of preparation.
If you take more Valcyte than you should
Contact your doctor or hospital immediately if you have taken, or think that you have taken, more Valcyte solution than you should. Taking more than the recommended dose can cause serious side effects, particularly affecting your blood or kidneys. You may need hospital treatment.
If you forget to take Valcyte
If you forget to take your dose of Valcyte take the missed dose as soon as you remember and take the next dose at the usual time. Do not take a double dose to make up for a missed dose.
If you stop taking Valcyte
You must not stop taking your medicine unless your doctor tells you to.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions
Up to 1 in every 100 people may have a sudden and severe allergic reaction to valganciclovir (anaphylactic shock). STOP taking Valcyte and go to the accident and emergency department at your nearest hospital if you experience any of the following:
• a raised, itchy skin rash (hives)
• sudden swelling of the throat, face, lips and mouth which may cause difficulty swallowing or breathing
• sudden swelling of the hands, feet or ankles
The side effects that have occurred during treatment with valganciclovir or ganciclovir are given below.
Very common side effects (may affect more than 1 in 10 users):
• Effects on the blood: a reduction in the number of white blood cells in the blood (neutropenia) - which will make you more likely to get infections, a reduction in the pigment in the blood that carries oxygen (anaemia) - which can cause tiredness and breathlessness when you exercise
• Effects on breathing: feeling short of breath or having trouble breathing (dyspnoea)
• Effects on the stomach and digestive system: diarrhoea
Common side effects (may affect 1 to 10 users in 100):
• Effects on the blood: a reduction in the number of leucocytes (blood cells that fight infection) in the blood (leucopenia), a reduction in the number of platelets in the blood (thrombocytopenia) - which can cause bruising and bleeding, a reduction in the number of several types of blood cells at the same time (pancytopenia)
• Effects on the nervous system: headache, difficulty sleeping (insomnia), strange tastes (dysgeusia), becoming less sensitive to touch
(hypoaesthesia), prickly or tingling skin (paraesthesia), loss of feeling in the hands or feet (peripheral neuropathy), dizziness, fits (convulsions)
• Effects in the eye: eye pain, swelling within the eye (oedema), separation of the inner lining of the eye (detached retina), seeing floaters
• Effects in the ear: earache
• Effects on breathing: coughing
• Effects on the stomach and digestion: feeling and being sick, stomach ache, constipation, wind, indigestion (dyspepsia), difficulty swallowing (dysphagia)
• Effects on the skin: inflamed skin (dermatitis), itching (pruritus), sweating at night
• Effects on the muscles, joints or bones: back pain, pain in the muscles (myalgia) or joints (arthralgia), stiff muscles (rigor), muscle cramps
• Infections: fungal infection in the mouth (oral candidiasis), infections caused by bacteria or viruses in the blood, inflammation of cellular tissue (cellulitis), inflammation or infection of the kidneys or bladder
• Effects in the liver: a rise in some liver enzymes, which will only be seen during blood tests
• Effects in the kidney: changes to the normal working of the kidneys
• Effects on eating: loss of appetite (anorexia), weight loss
• General effects: tiredness, fever, pain, chest pain, loss of energy (asthenia), generally feeling unwell (malaise)
• Effects on mood or behaviour: depression, feeling anxious, confused, having unusual thoughts
Uncommon side effects (may affect 1 to 10 users in 1,000):
• Effects in the heart: changes to the normal heart beat (arrhythmia)
• Effects on circulation: low blood pressure (hypotension), which can cause you to feel light headed or faint
• Effects on the blood: a decrease in the production of blood cells in the bone marrow
• Effects in the nerves: shaking or trembling (tremor)
• Effects in the eyes: red, swollen eyes (conjunctivitis), abnormal vision
• Effects in the ears: deafness
• Effects on the stomach or digestion: swollen stomach, mouth ulcers, inflammation of the pancreas (pancreatitis) where you may notice severe pain in the stomach and back
• Effects on the skin: hair loss (alopecia), itchy rash or swellings (urticaria), dry skin
• Effects in the kidneys: blood in the urine (haematuria), kidney failure
• Effects in the liver: a rise in the liver enzyme called alanine aminotransferase (which will only be seen during blood tests)
• Effects on fertility: infertility in men
• Effects on mood or behaviour: having unusual changes in mood and behaviour, losing contact with reality such as hearing voices or seeing things that are not there, feeling agitated
Rare side effects (may affect 1 to 10 users in 10,000):
• Effects on the blood: failure of the production of all types of blood cells (red blood cells, white blood cells and platelets) in the bone marrow
Separation of the inner lining of the eye (detached retina) has only happened in AIDS patients treated with Valcyte for CMV infection.
Additional side effects in children and adolescents
The side effects reported in children and adolescents are similar to the side effects reported for adults.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly (see details below). By reporting side effects you can help provide more information on the safety of this medicine.
Ireland
HPRA Pharmacovigilance Earlsfort Terrace IRL-Dublin 2 Tel: +353 1 6764971 Fax: +353 1 6762517 Website: www.hpra.ie e-mail: medsafetv@hpra.ie
Malta
ADR Reporting Website:
http://www.medicinesauthority.gov.mt/adrportal
United Kingdom
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard.
5. How to store Valcyte
Keep this medicine out of the sight and reach of children.
Do not use the powder after the expiry date which is stated on the carton and bottle label (EXP). The expiry date refers to the last day of that month.
Powder: does not require any special storage condition.
Reconstituted solution: Store in a refrigerator (2°C - 8°C).
The shelf-life of the oral solution is 49 days. Do not use the solution 49 days after preparation or after the expiry date which will be written on the bottle by the pharmacist.
Do not throw away medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What Valcyte contains
The active substance is valganciclovir hydrochloride. Following dissolution of the powder, 1 ml solution contains 55 mg valganciclovir hydrochloride corresponding to 50 mg valganciclovir as hydrochloride.
The other ingredients
(excipients) are: povidone,
fumaric acid, sodium
benzoate (E211), sodium
saccharin and mannitol,
tutti-frutti flavour
[maltodextrins (maize),
propylene glycol, arabic gum (E414) and
natural identical flavouring substances mainly
consisting of banana, pineapple and peach
flavour].
What Valcyte looks like and contents of the pack
Valcyte powder is a granulate with a white to slightly yellow colour. A quantity of 12 g powder is supplied in a glass bottle. Upon reconstitution, the volume of the solution is 100 ml, providing a usable volume of 88 ml. The solution is clear and colourless to brown. The pack also contains a bottle adapter and 2 dispensers that are graduated to 500 mg with 25 mg graduations.
Pack size: One bottle containing 12g powder.
Marketing Authorisation Holder
Roche Products Limited 6 Falcon Way Shire Park
Welwyn Garden City AL7 1TW United Kingdom
Manufacturer
Roche Pharma AG Emil-Barell-Str.1 D-79639 Grenzach-Wyhlen Germany
This medicinal product is authorised in the Member States of the EEA under the following names:
Valcyte: Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, The Netherlands,
Norway, Poland, Romania, Slovak Republic, Slovenia, Spain, Sweden, United Kingdom
RoValcyte: France, Portugal
This leaflet was last revised in January 2015
The following information is intended for healthcare professionals only:
It is recommended that the Valcyte solution be prepared by a pharmacist as follows:
1. Measure 91 ml of water in a graduated cylinder.
2. Remove the child resistant cap, add the water to the bottle, close the bottle with the child resistant cap and shake the closed bottle until the powder is dissolved.
3. Remove the child resistant cap and push the bottle adapter into the neck of the bottle.
4. Close the bottle with child resistant cap tightly to assure the proper seating of the bottle adapter in the bottle and child resistant status of the cap.
5. Write the date of expiration of the solution on the bottle label.
Avoid inhalation or direct contact of skin or mucous membranes with the powder and direct contact with the solution. If contact occurs, wash thoroughly with soap and water; rinse eyes with plain water.
10163234 GB