Vancomycin 500mg Powder For Solution For Infusion
PACKAGE LEAFLET INFORMATION FOR THE USER
VANCOMYCIN 500MG and 1G POWDER FOR SOLUTION FOR INFUSION
Vancomycin hydrochloride powder for solution for intravenous infusion or powder for solution for oral use
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have further questions, please ask your doctor or nurse.
- This medicine has been prescribed for you personally and you should not pass it on to others. It may harm them, even if their symptoms are the same as yours.
The name of your medicine is either vancomycin 500mg powder for solution for infusion or vancomycin 1g powder for solution for infusion. In the rest of this leaflet it is called vancomycin infusion.
In this leaflet:
1. What vancomycin infusion is and what it is used for
2. Before you are given vancomycin infusion
3. How vancomycin infusion is given
4. Possible side effects
5. How to store vancomycin infusion
6. Further information
1. WHAT VANCOMYCIN INFUSION IS AND WHAT IT IS USED FOR
Vancomycin is an antibiotic.
Vancomycin works by killing some types of bacteria that can cause infections in people. Like all antibiotics, vancomycin is only able to kill some types of bacteria so it is only suitable for treating some types of infection.
Vancomycin is used to treat severe infections caused by bacteria called ‘staphylococci’. These bacteria are resistant to many other antibiotics and vancomycin is used when other antibiotics may not be suitable. Vancomycin may be used for infections in the bone, lungs, heart, blood and skin.
It is sometimes necessary to give another antibiotic at the same time as vancomycin so as to be sure that all the types of bacteria causing an infection will be killed.
Vancomycin is sometimes given before an operation or a dental procedure to prevent you getting an infection.
If you are given this medicine as a liquid to swallow then it is being used to treat severe diarrhoea.
2. BEFORE YOU ARE GIVEN VANCOMYCIN INFUSION
Vancomycin infusion should not be given if you:
• have ever had an allergic reaction to vancomycin.
Speak to your doctor before vancomycin is given if you:
• have ever reacted badly to the antibiotic teicoplanin
• are hard of hearing. Your doctor may test your hearing during treatment, particularly if you are elderly
• have kidney problems or are having treatment (like dialysis) for kidney failure. You may need a lower dose of vancomycin infusion
• have ever had inflammation of the large bowel, called colitis or any other severe disease affecting your gut
• know you are going to have an operation. If you later have an operation then tell your surgeon that you have been given vancomycin.
If any of the above apply to you, speak to your doctor before you are given vancomycin infusion.
Taking other medicines
Taking another medicine while you are being given vancomycin infusion can affect how it or the other medicine works. Please inform your doctor or pharmacist if you are taking or have recently taken any other medicines, even those you may have bought yourself without a prescription.
Please check with your doctor if you are taking any of the following (or any other medication):
• tacrolimus or ciclosporin, drugs used to stop the body rejecting an organ after transplant
• dobutamine, a drug used to treat cardiac arrest or low blood pressure
• colestyramine, a drug used to lower cholesterol
• muscle relaxants such as suxamethonium
• loop diuretics such as furosemide
• anaesthetics (drugs which can put you to sleep)
• certain other antibiotics, used to treat infections (2.g. amphotericin B, aminoglycosides, bacitracin, polymixin B, collstin)
• cisplatin, a drug used to treat tumours Vancomycin must not be administered intramuscularly.
Pregnancy and breast-feeding
You should not be given vancomycin infusion if you are pregnant or think you might be pregnant unless you have discussed this with your doctor first.
Small amounts of vancomycin enter breast milk. If you cannot stop breast-feeding while you have vancomycin infusion, you should watch your baby carefully for any signs of diarrhoea or any other illness and tell your doctor if you notice anything wrong.
Driving and using machines
Vancomycin infusion has not been reported to affect your ability to drive or operate machinery.
3. HOW VANCOMYCIN INFUSION IS GIVEN
Vancomycin will be given by a doctor or nurse by injection into a “drip” into a vein in your arm (intravenous infusion). Vancomycin should be given over a period of at least one hour. Vancomycin must not be administered intramuscularly
Vancomycin infusion is supplied as a powder, so before it can be given it must be diluted and made into a solution. A doctor, nurse or pharmacist will do this.
Vancomycin may also be taken by mouth by dissolving the powder in a glass of water or squash.
Dosages
Vancomycin as an infusion Adults
The usual dose of vancomycin given as an infusion in adults is 500mg every 6 hours or 1g every 12 hours. The duration of treatment will depend on your infection and how you respond to vancomycin. If you have an infection of your heart you may require treatment for 3 weeks or longer.
Elderly
You may require a lower dose of vancomycin.
Pregnant women
You may require a higher dose of vancomycin.
Children
Over 1 month of age - The usual dose of vancomycin as an infusion in children is 10mg for
SUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE MEDICINAL PRODUCT
Vancomycin 500g Powder for Solution for Infusion Vancomycin 1 g Powder for Solution for Infusion
2. Qualitative and Quantitative Composition
Vancomycin 500mg Powder for Solution for Infusion - Each vial contains vancomycin
500mg* (equivalent to 500 000* IU) as vancomycin hydrochloride
Vancomycin 1g Powder for Solution for Infusion - Each vial contains vancomycin 1g*
(equivalent to 1 000 000* IU) as vancomycin hydrochloride
For full list of excipients, see 6.1
3. PHARMACEUTICAL FORM
Powder for solution for intravenous infusion Powder for solution for oral use ‘A white to cream coloured porous cake’
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
Vancomycin is indicated in potentially life-threatening infections which cannot be treated with other effective, less toxic antimicrobial drugs, including the penicillins and cephalosporins. Vancomycin is useful in the therapy of severe staphylococcal infections in patients who cannot receive or who have failed to respond to the penicillins and cephalosporins, or who have infections with staphylococci resistant to other antibiotics.
Vancomycin is used in the treatment of endocarditis and as prophylaxis against endocarditis in patients at risk from dental or surgical procedures.
Its effectiveness has been documented in other infections due to staphylococci, including osteomyelitis, pneumonia, septicaemia and soft tissue infections.
Vancomycin may be used orally for the treatment of staphylococcal enterocolitis and pseudomembranous colitis due to Clostridium difficile. Parenteral administration of vancomycin is not effective for these indications. Intravenous administration may be used concomitantly if required.
4.2 Posology and method of administration
For intravenous infusion and oral use only and not for intramuscular administration.
Please refer to Section 6.6 for full details on preparation.
Infusion-related adverse events are related to both concentration and rate of administration of vancomycin.
Concentrations of no more than 5mg/ml are recommended. In selected patients in need of fluid restriction, a concentration up to 10mg/ml may be used; use of such higher concentrations may increase the risk of infusion-related events. Infusions should be given over at least 60 minutes. In adults, if doses exceeding 500mg are used, a rate of infusion of no more than 10mg/min is recommended. Infusion-related events may occur, however, at any rate or concentration.
Intravenous infusion in patients with normal renal function Adults: The usual intravenous dose is 500mg every six hours or 1g every 12 hours, in sodium chloride intravenous infusion or 5% dextrose intravenous infusion. Each dose should be administered at no more than 10mg/min. Other patient factors, such as age, obesity or pregnancy, may call for modification of the usual daily dose. The majority of patients with infections caused by organisms sensitive to the antibiotic show a therapeutic response within 48-72 hours. The total duration of therapy is determined by the type and severity of the infection and the clinical response of the patient. In staphylococcal endocarditis, treatment for three weeks or longer is recommended.
Pregnancy: It has been reported that significantly increased doses may be required to achieve therapeutic serum concentrations in pregnant patients - Section 4.6 Pregnancy and lactation. The elderly: Dosage reduction may be necessary to a greater extent than expected because of decreasing renal function (see below). Monitor auditory function - see Section 4.4.Special warnings and precautions for use.
Children: The usual intravenous dosage is 10mg/kg per dose given every six hours (total daily dosage 40mg/kg of body weight). Each dose should be administered over a period of at least 60 minutes.
In neonates and young infants, the total daily dosage may be lower. An initial dose of 15mg/ kg is suggested, followed by 10mg/kg every 12 hours in the first week of life and every eight hours thereafter until one month of age. Each dose should be administered over 60 minutes. Close monitoring of serum vancomycin concentrations may be warranted in these patients. Patients with impaired renal function
Dosage adjustments must be made to avoid toxic serum levels. In premature infants and the elderly, greater dosage reductions than expected may be necessary because of decreased renal function. Regular monitoring of serum levels is advised in such patients, as accumulation has been reported, especially after prolonged therapy. Vancomycin serum concentrations may be determined by use of a microbiological assay, radioimmunoassay, fluorescence polarisation immunoassay, fluorescence immunoassay or high-pressure liquid chromatography. The following nomogram, based on creatinine clearance values, is provided.
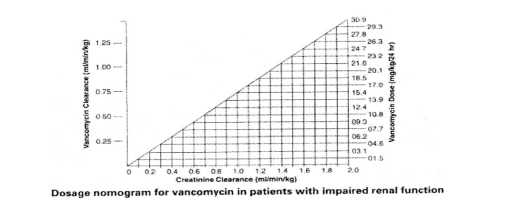
The nomogram is not valid for functionally anephric patients on dialysis. For such patients, a loading dose of 15mg/kg body weight should be given to achieve therapeutic serum levels promptly, and the dose required to maintain stable levels is 1.9mg/kg/24 hours. Since individual maintenance doses of 250mg to 1g are convenient, in patients with marked renal impairment a dose may be given every several days rather than on a daily basis. In anuria a dose of 1g every seven to ten days has been recommended.
For instructions on the preparation of solutions, See Section 6.6.
Measurement of serum concentrations
Following multiple intravenous doses, peak serum concentrations, measured two hours after infusion is complete, range from 18-26mg/l. Trough levels measured immediately prior to the next dose should be 5-10mg/l. Ototoxicity has been associated with serum drug levels of 80-100mg/l, but this is rarely seen when serum levels are kept at or below 30mg/l.
Oral administration
The contents of vials for parenteral administration may be used.
Adults and the elderly:The usual daily dose given is 500mg in divided doses for seven to ten days, although up to 2g/day have been used in severe cases. The total daily dosage should not exceed 2g. Each dose may be reconstituted in 30ml water and either given to the patient to drink, or administered by nasogastric tube.
Children: The usual daily dose is 40mg/kg in three or four divided doses for seven to ten days. The total daily dosage should not exceed 2g.
Common flavouring syrups may be added to the solution at the time of administration to improve the taste.
4.3 Contraindications
Hypersensitivity to vancomycin. Vancomycin must not be administered intramuscularly.
4.4 Special warnings and precautions for use Warnings
Rapid bolus administration (eg, over several minutes) may be associated with exaggerated hypotension, including shock, and, rarely, cardiac arrest. Vancomycin should be infused in a dilute solution over a period of not less than 60 minutes to avoid rapid infusion-related reactions. Stopping the infusion usually results in a prompt cessation of these reactions (see Section 4.2. Posology and method of administration and Section 4.8 Undesirable effects). Some patients with inflammatory disorders of the intestinal mucosa may have significant systemic absorption of oral vancomycin and, therefore, may be at risk for the development of adverse reactions associated with the parenteral administration of vancomycin. The risk is greater in patients with renal impairment. It should be noted that the total systemic and renal clearances of vancomycin are reduced in the elderly. Clearance of vancomycin is increased in burn victims
Due to its potential ototoxicity and nephrotoxicity, vancomycin should be used with care in patients with renal insufficiency and the dose should be reduced according to the degree of renal impairment. The risk of toxicity is appreciably increased by high blood concentrations or prolonged therapy or concomitant administration of other otoxic or nephrotoxic drugs. Blood levels should be monitored and renal function tests should be performed regularly. Vancomycin should also be avoided in patients with previous hearing loss. If it is used in such patients, the dose should be regulated, if possible, by periodic determination of the drug level in the blood. Tinnitus may precede the onset of deafness and necessitates discontinuation of vancomycin. The elderly are more susceptible to auditory damage. Experience with other antibiotics suggests that deafness may be progressive despite cessation of treatment.
Use in paediatrics: In premature neonates and young infants, it may be appropriate to confirm desired vancomycin serum concentrations. Concomitant administration of vancomycin and anaesthetic agents has been associated with erythema and histamine-like flushing in children. Use in the elderly: The natural decrement of glomerular filtration with increasing age may lead to elevated vancomycin serum concentrations if dosage is not adjusted (see Section 4.2 Posology and method of administration).
Precautions
Clinically significant serum concentrations have been reported in some patients being treated for active C. difficile-induced pseudomembranous colitis after multiple oral doses of vancomycin. Therefore, monitoring of serum concentrations may be appropriate in these patients.
Patients with borderline renal function and individuals over the age of 60 should be given serial tests of auditory function and of vancomycin blood levels. All patients receiving the drug should have periodic haematological studies, urine analysis and renal function tests. Vancomycin is very irritating to tissue, and causes injection site necrosis when injected intramuscularly; it must be infused intravenously and care must be taken to avoid extravasation. Injection site pain and thrombophlebitis occur in many patients receiving vancomycin and are occasionally severe.
The frequency and severity of thrombophlebitis can be minimised by administering the drug slowly as a dilute solution (2.5 to 5.0g/l) and by rotating the sites of infusion.
Prolonged use of vancomycin may result in the overgrowth of non-susceptible organisms.
Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken. In rare instances, there have been reports of pseudomembranous colitis, due to C. difficile, developing in patients who received intravenous vancomycin. Vancomycin should be used with caution in patients with teicoplanin sensitivity due to the possibility of cross-sensitivity.
4.5 Interaction with other medicinal products and other forms of interaction
Concomitant administration of tacrolimus with vancomycin has been associated with the potential for increased nephrotoxicity.
When used in conjunction with sympathomimetics dobabutamine or dopamine, vancomycin serum levels have the potential to be reduced. The clearance of vancomycin is enchanced by the sympathomimetics’ effect on cardiac output and renal blood flow.
If administered alongside the lipid-regulator colestyramine, the effects of oral vancomycin may be antagonized. Consequently, oral vancomycin should be administered 1 hour before or 4 to 6 hours after colestyramine.
Concomitant administration of vancomycin and anaesthetic agents has been associated with erythema, histamine-like flushing and anaphylactoid reactions.
|
Product Name |
Reference Number |
|
Vancomycin 500mg Powder for Solution for Infusion |
29831/0319 |
|
Vancomycin 1g Powder for Solution for Infusion |
29831/0322 |
This is a service provided by the Royal National Institute of Blind People
This leaflet was last revised in 08/2015
every kilogram of bodyweight every 6 hours. Children under 1 month of age require a lower dose.
Newborn (1st week of life) - The usual dose of vancomycin is 15mg for every kilogram of body weight followed by 10mg per kilogram of bodyweight every 12 hours.
Babies up to 1 month of age - The usual dose of vancomycin is 15mg for every kilogram of body weight followed by 10mg per kilogram of bodyweight every 8 hours.
If you have kidney problems
If you have kidney problems you will be given a lower dose of vancomycin.
Vancomycin given by mouth Adults
The usual dose of vancomycin given by mouth in adults is 500mg a day, divided into more than one dose, for seven to ten days. Up to 2g per day may be required.
Children
The usual dose of vancomycin given by mouth in children is 40mg for every kilogram of bodyweight each day, divided into three to four doses, for seven to ten days.
Blood tests
Your doctor may carry out blood tests during your treatment, particularly if you are elderly, a baby or child, pregnant, have kidney problems, or are being given vancomycin infusion by mouth for a severe bowel infection (colitis).
If you think you have missed a dose of vancomycin infusion
If you think you have missed a dose of vancomycin infusion, please tell your doctor or nurse.
If you think you have received too much vancomycin infusion
If you think you have received too much vancomycin infusion, please tell your doctor or nurse. If you stop receiving treatment with vancomycin infusion
As with all antibiotics it is important that you are given vancomycin infusion regularly and that the full course is completed. The treatment with vancomycin infusion should not be stopped unless advised by your doctor.
4. POSSIBLE SIDE EFFECTS
Like all medicines, vancomycin can cause side effects, although not everybody gets them. Vancomycin can cause an allergic reaction, with low blood pressure, a shock-like state and/or cardiac arrest, breathing difficulties, wheezing, rash and itchiness.
Rarely, a severe allergic reaction may occur with an extensive blistering or peeling skin rash, headache, fever, pain in the joints and sore eyes.
If you are given vancomycin by injection over a long period of time you may get severe diarrhoea.
If you think you are having an allergic reaction (see symptoms above) or develop a severe rash with blisters or severe diarrhoea, you must tell your doctor or nurse immediately.
If vancomycin is given into a vein too quickly, you may experience flushing of the neck, pain in the chest and back muscles, low blood pressure, slower heart beat and rarely your heart stopping. If you have any concerns please speak to a doctor or nurse.
Pain and irritation of the veins can occur at the site of injection.
Vancomycin can cause hearing loss. Tinnitus (noises in ears) may precede hearing loss. If you develop tinnitus tell doctor or nurse immediately.
Vancomycin can upset the normal working of the kidneys during treatment particularly if you are also taking an aminoglycoside antibiotic and this is usually picked up by abnormal blood test results.
Vancomycin can also reduce the numbers of different cells in the blood (symptoms can include
tiredness, sore throat, mouth ulcers, new infections and easy bruising or bleeding).
Other side effects include hearing loss, dizziness, giddiness, ringing in the ears, feeling sick, drug fever (fever resulting from an allergic reaction to a drug, that clears when treatment is stopped) and chills.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE VANCOMYCIN INFUSION
Keep your medicine out of the reach and sight of children.
Your doctor, nurse or pharmacist will usually be responsible for storing and preparing vancomycin before use and for checking that the vials have not passed their expiry date. The vials will be stored below 25°C. Made-up solutions may be stored for up to 24 hours in a refrigerator (2°C - 8°C).
The medicine must not be used after the expiry date which is stated on the carton and the label. The expiry date refers to the last day of that month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What vancomycin infusion contains
Each vial contains the active substance vancomycin (as vancomycin hydrochloride), as a powder for solution for intravenous infusion or powder for solution for oral use.
There are no other ingredients included in the product.
What vancomycin infusion looks like and contents of the pack
Vancomycin infusion comes in a glass vial with a rubber stopper capped with a flip-off cap. The powder is a white to cream coloured solid.
Each 500mg vial contains 500,000IU vancomycin. Each 1g vial contains 1,000,000IU vancomycin.
It is available in packs of one, two, five or ten vials. Not all pack sizes may be marketed. Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder: Wockhardt UK Limited, Ash Road North, Wrexham, LL13 9UF, UK. Manufacturer: CP Pharmaceuticals Ltd, Ash Road North, Wrexham, LL13 9UF, UK.
Other formats:
To listen to or request a copy of this leaflet in Braille, large print or audio please call, free of charge: 0800 198 5000 (UK Only)
Please be ready to give the following information:
^WOCKHARDT
>4
Twockhardt
There have been reports that the frequency of infusion-related events increases with the concomitant administration of anaesthetic agents. Infusion-related events may be minimised by the administration of vancomycin as a 60-minute infusion prior to anaesthetic induction. Concurrent or sequential systemic or topical use of other potentially neurotoxic, nephrotoxic, or ototoxic drugs, such as amphotericin B, aminoglycosides, bacitracin, polymixin B, colistin, viomycin or cisplatin, when indicated, requires careful monitoring.
There is a potential for an increase in nephrotoxicity when vancomycin in given with ciclosporin.
Concomitant use of vancomycin with muscle relaxants suxamethonium or vecuronium has been associated with the potential for enhanced neuromuscular blockade.
There is a potential for increased ototoxicity when vancomycin and loop diuretics are administered concomitantly. Caution should be used when administering vancomycin with furosemide, as there is a potential for furosemide, alone or in combination with theophylline, to reduce vancomycin serum levels.
4.6 Pregnancy and lactation
Pregnancy: A moderate amount of data on pregnant women pregnancy outcomes indicates no malformative or feto/neonatal toxicity. In a controlled clinical study, the potential ototoxic and nephrotoxic effects of vancomycin hydrochloride on infants were evaluated when the drug was administered to pregnant women for serious staphylococcal infections complicating intravenous drug abuse. Vancomycin hydrochloride was found in cord blood. No sensorineural hearing loss or nephrotoxicity attributable to vancomycin was noted. One infant, whose mother received vancomycin in the third trimester, experienced conductive hearing loss that was not attributable to vancomycin. Because vancomycin was administered only in the second and third trimesters, it is not known whether it causes foetal harm. Animal studies do not indicate reproductive toxicity (see section 5.3). The use of vancomycin may be considered during pregnancy if necessary. Blood levels should be monitored carefully to minimise the risk of foetal toxicity. It has been reported, however, that pregnant patients may require significantly increased doses of vancomycin to achieve therapeutic serum concentrations. Breast-feeding: Vancomycin is excreted in human milk, but at therapeutic doses of vancomycin no effects on the breastfed newborns/infants are anticipated. Caution should be exercised when vancomycin is administered to a nursing woman. It is unlikely that a nursing infant can absorb a significant amount of vancomycin from its gastro-intestinal tract.
4.7 Effects on ability to drive and use machines Not applicable
4.8 Undesirable effects
Blood and lymphatic system disorders. Reversible neutropenia, usually starting one week or more after onset of intravenous therapy or after a total dose of more than 25g. Neutropenia appears to be promptly reversible when vancomycin is discontinued. Thrombocytopenia has rarely been reported. Reversible agranulocytosis (less than 500 granulocytes per mm3) has been reported rarely, although causality has not been established.
Immune system disorders: hypersensitivity reactions, anaphylaxis, and drug fever Ear and labyrinth disorders: Hearing loss associated with intravenously administered vancomycin has been reported and may be transient or permanent. Tinnitus may precede the onset of deafness and necessitates discontinuation of vancomycin. Ototoxicity is most likely to occur in patients with kidney dysfunction, pre-existing hearing loss or concomitant treatment with an ototoxic drug. Vertigo and dizziness have been reported rarely. Gastrointestinal disorders. Rare, potential for pseudomembranous colitis, associated with Clostridium difficile, to occur with vancomycin. Nausea
Vascular disorders: Plebitis, chills and rare cases of vasculitis Skin and subcutaneous tissue disorders, eosinophilia, rashes (including exfoliative dermatitis), Vancomycin has been associated with the bullous eruption disorders, Stevens-Johnson syndrome, toxic epidermal necrolysis and linear IgA bullous dermatosis. If a bullous disorder is suspected, the drug should be discontinued and specialist dermatological assessment should be carried out. Dermatitis ascribed to specific agent. Cases of Drug Rash with Eosinophilia and Systemic Symptoms (DRESS) have been reported.
Renal and urinary disorders. Rarely, renal failure, principally manifested by increased serum creatinine or blood urea concentrations, have been observed, especially in patients given large doses of intravenously administered vancomycin. Rare cases of interstitial nephritis have been reported. Most occurred in patients who were given aminoglycosides concomitantly or who had pre-existing kidney dysfunction. When vancomycin was discontinued, azotaemia resolved in most patients.
General disorders and administration site conditions. Infusion-related events: During or soon after rapid infusion of vancomycin, patients may develop anaphylactoid reactions with hypotension, a shock-like state and/or cardiac arrest, wheezing, dyspnoea, urticaria or pruritus. Rapid infusion may also be associated with pain and muscle spasm of the chest and back or the red neck or red man syndrome (characterised by erythema, flushing or rash over the face, neck, and upper torso accompanied by a sudden drop in blood pressure). These reactions usually resolve within 20 minutes but may persist for several hours. In animal studies, hypotension and bradycardia occurred in animals given large doses of vancomycin at high concentrations and rates. Such events are infrequent if vancomycin is given by slow infusion over 60 minutes. In studies of normal volunteers, infusion-related events did not occur when vancomycin was administered at a rate of 10mg/min or less.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
4.9 Overdose
Supportive care is advised, with maintenance of glomerular filtration. Vancomycin is poorly removed from the blood by haemodialysis or peritoneal dialysis. Haemoperfusion with Amberlite resin XAD-4 has been reported to be of limited benefit. Haemofiltration effectively removes vancomycin from the blood.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
ATC Code: JO1X A (Glycopeptide antibacterial)
Vancomycin is a glycopeptide antibiotic derived from Nocardia orientals (formerly Streptomyces orientals), and is active against many Gram-positive bacteria, including
Staphylococcus aureus, Staph, epidermidis, alpha and beta haemolytic streptococci, group D streptococci, corynebacteria and clostridia.
5.2. Pharmacokinetic properties
Vancomycin is not significantly absorbed from the normal gastro-intestinal tract and is therefore not effective by the oral route for infections other than staphylococcal enterocolitis and pseudomembranous colitis due to Clostridium difficile.
5.3 Preclinical safety data
Although no long-term studies in animals have been performed to evaluate carcinogenic potential, no mutagenic potential of vancomycin was found in standard laboratory tests. Reproduction studies in rats and rabbits at doses up to 1 and 1.1 times the maximum recommended human dose (MRHD) based on body surface area, respectively, have revealed no teratogenic effects. No effects on foetal weight or development were seen with the same doses in rats or slightly lower doses in rabbits (0.74 times the MRHD). No other definitive fertility studies have been performed.
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients None
6.2 Incompatibilities
Vancomycin solution has a low pH that may cause chemical or physical instability when it is mixed with other compounds.
This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6.
6.3 Shelf life Unopened - 36 months
Reconstituted solution intended for parenteral administration
Physical and chemical stability have been demonstrated for a period of 24 hours when stored at 2° to 8°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless reconstitution and dilution has taken place in controlled and validated aseptic conditions.
Prior to administration, parenteral drug products should be inspected visually for particulate matter and discolouration whenever solution or container permits.
Reconstituted solution intended for oral administration
Solution intended for oral administration may be stored in a refrigerator (2° to 8°C) for up to 24 hours.
6.4 Special precautions for storage
Unopened: Do not store above 25°C
After reconstitution: Store at 2-8°C (see 6.3 Shelf Life).
6.5 Nature and contents of container Vancomycin 500g Powder for Infusion:-
Packs* of one, two, five or ten Type II colourless glass 10ml vials stoppered with Type I rubber stopper, capped with a flip-off cap.
Vancomycin 1g Powder for Infusion:-
Packs* of one, two, five or ten Type II colourless glass 20ml vials stoppered with Type I rubber stopper, capped with a flip-off cap. *Not all pack sizes may be marketed
6.6 Special precautions for disposal
Preparation of solution. At the time of use, add 10ml of water for Injections to the 500mg vial. And add 20ml of water for injections to the 1g vial. Vials reconstituted in this manner will give a solution of 50mg/ml. The reconstituted solution is clear and colourless.
Further dilution is required. Read instructions which follow:
1. Intermittent infusion is the preferred method of administration. Reconstituted solutions containing 500mg vancomycin must be diluted with at least 100ml diluent. Reconstituted solutions containg1g vancomycin must be diluted with at least 200ml diluent. 0.9% sodium chloride intravenous infusion or 5% dextrose intravenous infusion are suitable diluents. The desired dose should be given by intravenous infusion over a period of at least 60 minutes.
If administered over a shorter period of time or in higher concentrations, there is the possibility of inducing marked hypotension in addition to thrombophlebitis. Rapid administration may also produce flushing and a transient rash over the neck and shoulders.
2. Continuous infusion (should be used only when intermittent infusion is not feasible). 1-2g can be added to a sufficiently large volume of sodium chloride intravenous infusion or 5% dextrose intravenous infusion to permit the desired daily dose to be administered slowly by intravenous drip over a 24 hour period.
3. Oral administration
The contents of vials for parenteral administration may be used.
Common flavouring syrups may be added to the solution at the time of administration to improve the taste.
Vials are for single use only and any unused product or waste material should be disposed of immediately in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Wockhardt UK Limited, Ash Road North, Wrexham LL13 9UF, United Kingdom
8. MARKETING AUTHORISATION NUMBER
Vancomycin 500mg Powder for Infusion - PL 29831/0319 Vancomycin 1g Powder for Infusion - PL 29831/0322
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
4 April 2008
10. DATE OF REVISION OF THE TEXT:
08/2015
103385/5