Ventolin Solution For Intravenous Infusion
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
VentolinTM Solution for Intravenous Infusion 5mg in 5ml (1mg/ml).
2. Qualitative and Quantitative Composition
Ventolin Solution for Intravenous Infusion 5mg in 5ml (1mg/ml) is presented as ampoules of 5ml, each containing 5mg salbutamol as Salbutamol Sulfate BP in a sterile isotonic solution.
3 PHARMACEUTICAL FORM
Clear, colourless or pale straw-coloured solution for intravenous infusion.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Ventolin Solution for Intravenous Infusion is indicated in adults and adolescents.
Ventolin Solution for Intravenous Infusion should be administered under the direction of a physician. It is indicated for two distinct clinical situations:
a) For the relief of severe bronchospasm.
b) For the short term management of uncomplicated premature labour; to arrest labour between 22 and 37 weeks of gestation in patients with no medical or obstetric contraindication to tocolytic therapy.
4.2 Posology and method of administration
Ventolin Solution for Intravenous Infusion is used to prepare an infusion solution. It should not be injected undiluted. Ventolin Solution for Intravenous Infusion should not be administered in the same syringe or infusion as any other medication.
1. In severe bronchospasm.
Adults: A suitable solution for infusion providing 10 micrograms salbutamol/ml is prepared by diluting 5ml Ventolin Solution for Intravenous Infusion to 500ml with an infusion solution such as Sodium Chloride and
Dextrose Injection BP. Other suitable diluents are Water for Injections BP, Sodium Chloride Injection BP or Dextrose Injection BP.
Infusion rates providing 3 to 20 micrograms salbutamol/minute (0.3 to 2ml/minute of the above infusion solution) are usually adequate. Higher doses have been used with success in patients with respiratory failure.
Paediatric Population
The safety and efficacy of Ventolin Solution for Intravenous in children under the age of 12 has not been established. From the available data no recommendation on posology can be made.
Children aged 12 years and over: Dose as per adult population
2. In the short term management of uncomplicated premature labour.
Treatment with Ventolin Solution for Intravenous Infusion should only be initiated by obstetricians/physicians experienced in the use of tocolytic agents. It should be carried out in facilities adequately equipped to perform continuous monitoring of maternal and foetus health status.
Duration of treatment should not exceed 48 hours as data show that the main effect of tocolytic therapy is a delay in delivery of up to 48 hours; no statistically significant effect on perinatal mortality or morbidity has been observed in randomised, controlled trials. This short term delay may be used to implement other measures known to improve perinatal health.
Ventolin Solution for Intravenous Infusion should be administered as early as possible after the diagnosis of premature labour, and after evaluation of the patient to eliminate any contra-indications to the use of salbutamol (see section 4.3). This should include an adequate assessment of the patient's cardiovascular status with supervision of cardiorespiratory function and ECG monitoring throughout treatment (see section 4.4).
Special cautions for infusion: The dose must be individually titrated with reference to suppression of contractions, increase in pulse rate and changes in blood pressure, which are limiting factors. These parameters should be carefully monitored during treatment. A maximum maternal heart rate of 120 beats per min should not be exceeded.
Careful control of the level of hydration is essential to avoid the risk of maternal pulmonary oedema (see section 4.4). The volume of fluid in which the drug is administered should thus be kept to a minimum. A controlled infusion device should be used, preferably a syringe pump.
Infusion rates providing 10 to 45 micrograms salbutamol/minute are generally adequate to control uterine contractions. A starting rate of 10 micrograms/minute is recommended, increasing the rate at 10-minute intervals until there is evidence of patient response shown by diminution in strength, frequency or duration of contractions. Thereafter, the infusion rate may be increased slowly until contractions cease. Careful attention should be given to cardio-respiratory function and fluid balance monitored. Once uterine contractions have ceased the infusion rate should be maintained at the same level for one hour and then reduced by 50% decrements at six hourly intervals. If labour progresses despite treatment the infusion should be stopped.
Dilution: The recommended diluent is 5% Dextrose (see section 4.3 for precautions with diabetic patients).
For use in a syringe pump: Prepare a solution providing 200 micrograms salbutamol/ml by diluting 10ml Ventolin Solution for Intravenous Infusion with 40ml diluent. An infusion rate of 10 to 45 micrograms/minute is equivalent to 0.05 to 0.225ml/minute of this solution.
Other infusion methods: Prepare a solution providing 20 micrograms salbutamol/ml by diluting 10ml Ventolin Solution for Intravenous Infusion with 490ml diluent. An infusion rate of 10 to 45 micrograms/minute is equivalent to 0.5 to 2.25ml/minute of this solution.
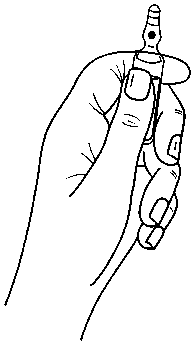
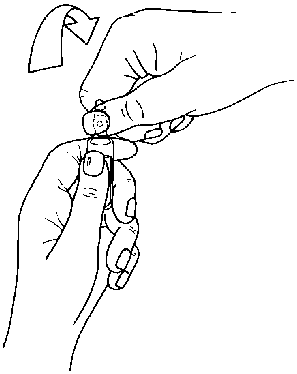
Instructions to open the ampoule
Ampoules are equipped with the OPC (One Point Cut) opening system and must be opened using the following instructions:
• hold with one hand the bottom part of the ampoule as indicated in Picture 1
• put the other hand on the top of the ampoule positioning the thumb above the coloured point and press as indicated in Picture 2
Picture 1
Picture 2
4.3 Contraindications
Ventolin Solution for Intravenous Infusion is contra-indicated in the following conditions:
- Any condition at a gestational age < 22 weeks
- as a tocolytic agent in patients with pre-existing ischaemic heart disease or those patients with significant risk factors for ischaemic heart disease.
- threatened abortion during the 1st and 2nd trimester
- any condition of the mother or foetus in which prolongation of the pregnancy is hazardous, e.g. severe toxaemia, intrauterine infection, vaginal bleeding resulting from placenta praevia, eclampsia or severe preeclampsia, placental abruption, or cord compression.
- intrauterine foetal death, known lethal congenital or lethal chromosomal malformation.
Ventolin Solution for Intravenous Infusion is also contraindicated in any preexisting medical conditions with which a betamimetic would have an untoward effect e.g., pulmonary hypertension and cardiac disorders such as hypertrophic obstructive cardiomyopathy or any type of obstruction of the left ventricular outflow tract, e.g. aortic stenosis.
Hypersensitivity to the active substance or any of the excipients listed in section 6.1.
Non-i.v. formulations of salbutamol must not be used to arrest uncomplicated premature labour or threatened abortion.
4.4 Special warnings and precautions for use
Bronchodilators should not be the only or main treatment in patients with severe or unstable asthma. Severe asthma requires regular medical assessment, including lung-function testing, as patients are at risk of severe attacks and even death. Physicians should consider using the maximum recommended dose of inhaled corticosteroid and/or oral corticosteroid therapy in these patients.
The dosage or frequency of administration should only be increased on medical advice.
Patients being treated with Ventolin Solution for Intravenous Infusion may also be receiving short-acting inhaled bronchodilators to relieve symptoms. Increasing use of bronchodilators, in particular short-acting inhaled P2-agonists to relieve symptoms, indicates deterioration of asthma control.
The patient should be instructed to seek medical advice if short-acting relief bronchodilator treatment becomes less effective, or more inhalations than usual are required. In this situation the patient should be assessed and consideration given to the need for increased anti-inflammatory therapy (e.g. higher doses of inhaled corticosteroid or a course of oral corticosteroid).
Severe exacerbations of asthma must be treated in the normal way. The use of Ventolin Solution for Intravenous Infusion in the treatment of severe bronchospasm does not obviate the requirement for corticosteroid therapy as appropriate. When practicable, administration of oxygen concurrently with parenteral Ventolin is recommended, particularly when it is given by intravenous infusion to hypoxic patients. In common with other P-adrenoceptor agonists, salbutamol can induce reversible metabolic changes such as hypokalaemia and increased blood glucose levels. Diabetic patients may be unable to compensate for the increase in blood glucose and the development of ketoacidosis has been reported. Concurrent administration of corticosteroids can exaggerate this effect.
Therefore, diabetic patients and those concurrently receiving corticosteroids should be monitored frequently during intravenous infusion of Ventolin so that remedial steps (e.g. an increase in insulin dosage) can be taken to counter any metabolic change occurring. For these patients it may be preferable to dilute Ventolin Solution for Intravenous Infusion in Sodium Chloride Injection BP rather than in diluents containing dextrose.
Cardiovascular effects may be seen with sympathomimetic drugs, including salbutamol. There is some evidence from post-marketing data and published literature of myocardial ischaemia associated with salbutamol.
Tocolysis
Any decision to initiate therapy with Ventolin Solution for Intravenous Infusion should be undertaken after careful consideration of the risks and benefits of treatment.
Treatment should only be carried out in facilities adequately equipped to perform continuous monitoring of maternal and foetal health status. Tocolysis with beta-agonists is not recommended when membranes have ruptured or the cervix dilation is beyond 4cm.
Ventolin Solution for Intravenous Infusion should be used with caution in tocolysis and supervision of cardiorespiratory function and ECG monitoring, should be performed throughout treatment. The following monitoring measures must be constantly applied to the mother and, when feasible/appropriate, to the foetus:
- blood pressure and heart rate
- ECG
- electrolyte and fluid balance - to monitor for pulmonary oedema
- glucose and lactate levels - with particular regard to diabetic patients
- potassium levels- beta-agonists are associated with a decrease in serum potassium which increases the risk of arrhythmias (see section 4.5)
Treatment should be discontinued if signs of myocardial ischaemia (such as chest pain or ECG changes) develop.
Ventolin Solution for Intravenous Infusion should not be used as a tocolytic agent in patients with significant risk factors for, or a suspicion of any kind of pre-existing heart disease (e.g. tachyarrhythmias, heart failure, or valvular heart disease; see section 4.3). In premature labour in a patient with known or suspected cardiac disease, a physician experienced in cardiology should assess the suitability of treatment before intravenous infusion with Ventolin Solution for Intravenous Infusion.
Pulmonary oedema
As maternal pulmonary oedema and myocardial ischaemia have been reported during or following treatment of premature labour with beta-agonists, careful attention should be given to fluid balance and cardio-respiratory function. Patients with predisposing factors including multiple pregnancies, fluid overload, maternal infection and pre-eclampsia may have an increased risk of developing pulmonary oedema. Administration with a syringe pump as opposed to i.v. infusion will limit risk of fluid overload. If signs of pulmonary oedema or myocardial ischaemia develop, discontinuation of treatment should be considered (see section 4.2 and 4.8).
Blood pressure and heart rate
Increases in maternal heart rate of the order of 20 to 50 beats per minute usually accompany infusion of beta-agonists. The maternal pulse rate should be monitored and the need to control such increases by dose reduction or drug withdrawal should be evaluated on a case by case basis.
Generally maternal pulse rate should not be allowed to exceed a steady rate of 120 beats per minute.
Maternal blood pressure may fall slightly during the infusion; the effect being greater on diastolic than on systolic pressure. Falls in diastolic pressure are usually within the range of 10 to 20mmHg. The effect of infusion on foetal heart rate is less marked, but increases of up to 20 beats per minute may occur.
In order to minimise the risk of hypotension associated with tocolytic therapy, special care should be taken to avoid caval compression by keeping the patient in the left or right lateral positions throughout the infusion.
Diabetes
Administration of beta agonists is associated with a rise of blood glucose. Therefore blood glucose and lactate levels should be monitored in mothers with diabetes and diabetic treatment adjusted accordingly to meet the needs of the diabetic mother during tocolysis (see section 4.5).
Hyperthyroidism
Ventolin Solution for Intravenous Infusion should only be administered cautiously to patients suffering from thyrotoxicosis after careful evaluation of the benefits and risks of treatment.
Respiratory indications
Patients with underlying severe heart disease (e.g. ischaemic heart disease, arrhythmia or severe heart failure) who are receiving salbutamol should be warned to seek medical advice if they experience chest pain or other symptoms of worsening heart disease. Attention should be paid to assessment of symptoms such as dyspnoea and chest pain, as they may be of either respiratory or cardiac origin.
Salbutamol should be administered cautiously to patients suffering from thyrotoxicosis.
Potentially serious hypokalaemia may result from ^-agonist therapy, mainly from parenteral and nebulised administration. Particular caution is advised in acute severe asthma as this effect may be potentiated by hypoxia and by concomitant treatment with xanthine derivatives, steroids and diuretics.
Serum potassium levels should be monitored in such situations.
Lactic acidosis has been reported in association with high therapeutic doses of intravenous and nebulised short-acting beta-agonist therapy, mainly in patients being treated for an acute asthma exacerbation (see Section 4.8). Increase in lactate levels may lead to dyspnoea and compensatory hyperventilation, which could be misinterpreted as a sign of asthma treatment failure and lead to inappropriate intensification of short-acting beta-agonist treatment. It is therefore recommended that patients are monitored for the development of elevated serum lactate and consequent metabolic acidosis in this setting.
4.5 Interaction with other Medical Products and other forms of Interaction
Ventolin Solution for Intravenous Infusion should not be administered in the same syringe or infusion as any other medication.
Salbutamol and non-selective ^-blocking drugs such as propranolol, should not usually be prescribed together.
Halogenated anaesthetics
Owing to the additional antihypertensive effect, there is increased uterine inertia with risk of haemorrhage; in addition, serious ventricular rhythm disorders due to increased cardiac reactivity, have been reported on interaction with halogenated anaesthetics. Treatment should be discontinued, whenever possible, at least 6 hours before any scheduled anaesthesia with halogenated anaesthetics.
Corticosteroids
Systemic corticosteroids are frequently given during premature labour to enhance foetal lung development. There have been reports of pulmonary oedema in women concomitantly administered with beta-agonists and corticosteroids.
Corticosteroids are known to increase blood glucose and can deplete serum potassium, therefore concomitant administration should be undertaken with caution with continuous patient monitoring owing to the increased risk of hyperglycaemia and hypokalaemia (see section 4.4).
Anti-diabetics
The administration of beta-agonists is associated with a rise of blood glucose, which can be interpreted as an attenuation of anti-diabetic therapy; therefore individual anti-diabetic therapy may need to be adjusted (see section 4.4).
Potassium depleting agents
Owing to the hypokalaemic effect of beta-agonists, concurrent administration of serum potassium depleting agents known to exacerbate the risk of hypokalaemia, such as diuretics, digoxin, methyl xanthines and corticosteroids, should be administered cautiously after careful evaluation of the benefits and risks with special regard to the increased risk of cardiac arrhythmias arising as a result of hypokalaemia (see section 4.4).
4.6 Pregnancy and Lactation
Pregnancy
Administration of drugs during pregnancy should only be considered if the expected benefit to the mother is greater than any possible risk to the fetus. As with the majority of drugs, there is little published evidence of the safety of salbutamol in the early stages of human pregnancy, but in animal studies there was evidence of some harmful effects on the fetus at very high dose levels.
Breast-feeding
As salbutamol is probably secreted in breast milk, its use in nursing mothers requires careful consideration. It is not known whether salbutamol has a harmful effect on the neonate, and so its use should be restricted to situations where it is felt that the expected benefit to the mother is likely to outweigh any potential risk to the neonate.
Fertility
There is no information on the effects of salbutamol on human fertility. There were no adverse effects on fertility in animals (see section 5.3).
4.7. Effects on Ability to Drive and Use Machines
None reported.
4.8 Undesirable Effects
Adverse events are listed below by system organ class and frequency. Frequencies are defined as: very common (>1/10), common (>1/100 and <1/10), uncommon (>1/1000 and <1/100), rare (>1/10,000 and <1/1000) and very rare (<1/10,000). Very common and common events were generally determined from clinical trial data. Rare, very rare and unknown events were generally determined from spontaneous data.
The most common undesirable effects of Ventolin Solution for Intravenous Infusion are correlated with the betamimetic pharmacological activity and may be limited or avoided by a close monitoring of hemodynamic parameters, such as blood pressure and heart rate, and an appropriate adjustment of the dose. They normally recede upon therapy discontinuation.
Immune system disorders
Very rare: Hypersensitivity reactions including angioedema,
urticaria, bronchospasm, hypotension and collapse.
Metabolism and nutrition disorders
Common: *Hypokalaemia.
Rare: 'Hypokalaemia
Rare: *Hyperglycaemia
Unknown: Lactic acidosis (see section 4.4)
Nervous system disorders
Very common: Tremor.
Common: Headache.
Very rare: Hyperactivity.
Cardiac disorders
Very common: Very common:
'Tachycardia.
*Tachycardia.
'Palpitations
Common:
Common:
*Palpitations, *decrease in diastolic pressure
Rare: Cardiac arrhythmias e.g. atrial fibrillation,
supraventricular tachycardia and extrasystoles.
Rare: *Cardiac arrhythmias e.g. atrial fibrillation,
*Myocardial ischaemia (see section 4.4)
Vascular disorders
Common: *Hypotension (see section 4.4)
Rare: 'Peripheral vasodilatation.
Rare: *Peripheral vasodilatation.
Respiratory, thoracic and mediastinal disorders
Uncommon: 'Pulmonary oedema.
Uncommon: * Pulmonary oedema.
Gastrointestinal disorders
Unknown: Nausea, vomiting.
In the management of premature labour, intravenous infusion of Ventolin has very rarely been associated with nausea and vomiting.
Musculoskeletal and connective tissue disorders
Common: Muscle cramps.
* These reactions have been reported in association with the use of short acting beta-agonists in obstetric indications and are considered class effects (see section 4.4).
' Frequency associated with bronchospasm indication, where the reaction has also been seen in the obstetric indication.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
The most common signs and symptoms of overdose with salbutamol are transient beta agonist pharmacologically mediated events, including tachycardia, tremor, hyperactivity and metabolic effects including hypokalaemia and lactic acidosis (see sections 4.4 and 4.8).
Hypokalaemia may occur following overdose with salbutamol. Serum potassium levels should be monitored.
Lactic acidosis has been reported in association with high therapeutic doses as well as overdoses of short-acting beta-agonist therapy, therefore monitoring for elevated serum lactate and consequent metabolic acidosis (particularly if there is persistence or worsening of tachypnea despite resolution of other signs of bronchospasm such as wheezing) may be indicated in the setting of overdose.
Nausea, vomiting and hyperglycaemia have been reported, predominantly in children and when salbutamol overdose has been taken via the oral route.
Further management should be as clinically indicated or as recommended by the national poisons centre, where available.
5.1 Pharmacodynamic Properties
Pharmacotherapeutic group: Selective beta-2-adrenoreceptor agonists ATC Code: R03CC02
Salbutamol is a selective beta-2 andrenoceptor agonist which acts on the beta-2
adrenoceptors of the bronchi and uterus. At therapeutic doses it acts on the beta-2 adrenoceptors of bronchial muscle providing short acting (4 to 6 hour) bronchodilation in reversible airways obstruction.
5.2 Pharmacokinetic Properties
Salbutamol administered intravenously has a half-life of 4 to 6 hours and is cleared partly renally and partly by metabolism to the inactive 4'-0-sulfate (phenolic sulfate) which is also excreted primarily in the urine. The faeces are a minor route of excretion. Most of a dose of salbutamol given intravenously, orally or by inhalation is excreted within 72 hours. Salbutamol is bound to plasma proteins to the extent of 10%.
5.3 Preclinical Safety Data
In common with other potent selective P2-agonists, salbutamol has been shown to be teratogenic in mice when given subcutaneously. In a reproductive study, 9.3% of fetuses were found to have cleft palate at 2.5mg/kg dose, 4 times the maximum human oral dose. In rats, treatment at the levels of 0.5, 2.32, 10.75 and 50mg/kg/day orally throughout pregnancy resulted in no significant fetal abnormalities. The only toxic effect was an increase in neonatal mortality at the highest dose level as the result of lack of maternal care. Reproductive studies in the rabbit at doses of 50mg/kg/day orally (i.e.
much higher than the normal human dose) have shown fetuses with treatment related changes; these included open eyelids (ablepharia), secondary palate clefts (palatoschisis), changes in ossification of the frontal bones of the cranium (cranioschisis) and limb flexure.
In an oral fertility and general reproductive performance study in rats at doses of 2 and 50 mg/kg/day, with the exception of a reduction in number of weanlings surviving to day 21 post partum at 50 mg/kg/day, there were no adverse effects on fertility, embryofetal development, litter size, birth weight or growth rate.
6.1 List of Excipients
Sodium chloride, sodium hydroxide, sulfuric acid and Water for Injections.
6.2. Incompatibilities
None stated.
6.3. Shelf-Life
36 months.
24 hours after mixing with infusion fluids.
6.4 Special Precautions For Storage
Store below 30°C and keep container in the outer carton in order to protect from light.
6.5. Nature and Contents of Container
Clear, 5ml neutral glass ampoules, available in boxes of 10 ampoules or 5 ampoules.
6.6 Special precautions for disposal and other handling
Ventolin Solution for Intravenous Infusion must be diluted before use. The recommended diluents are Water for Injections BP, Sodium Chloride Injection BP, Sodium Chloride and Dextrose Injection BP and Dextrose Injection BP (see section 4.2).
All unused admixtures of Ventolin Solution for Intravenous Infusion with infusion fluids should be discarded twenty-four hours after preparation.
7 MARKETING AUTHORISATION HOLDER
Glaxo Wellcome UK Ltd trading as GlaxoSmithKline UK Stockley Park West Uxbridge
Middlesex UB11 1BT.
8. MARKETING AUTHORISATION NUMBER(S)
PL 10949/0087
9. DATE OF FIRST AUTHORISATION / RENEWAL OF THE AUTHORISATION
20 October 1995/11 September 2000
10 DATE OF REVISION OF THE TEXT
02/03/2015