Zaditen 0.25mg/Ml Eye Drops Solution
Out of date information, search anotherRef: 0922/121114/1 /F
(ketotifen)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only.
Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Your medicine is called Zaditen 0.25mg/ml Eye Drops Solution but will be referred to as Zaditen throughout the leaflet.
What is in this leaflet:
What Zaditen is and what it is used for What you need to know before you use Zaditen
How to use Zaditen Possible side effects 3 How to store Zaditen
Contents of the pack and other information
Zaditen contains benzalkonium chloride
Zaditen contains benzalkonium chloride and may cause eye irritation.
If you wear soft contact lenses you should remove
them before using Zaditen as it can
discolour your soft contact lenses. You should wait
at least 15 minutes after using Zaditen
before reinserting your contact lenses into your
eyes.
^ What Zaditen is and what it is used for
Zaditen contains the active substance ketotifen, which is an anti-allergic substance.
Zaditen is used to treat eye symptoms of hay fever.
What you need to know before you use Zaditen
Do not use Zaditen
If you are allergic (hypersensitive) to ketotifen or any of the other ingredients of this medicine (listed in section 6).
Other medicines and Zaditen
If you need to apply any other medicinal products to your eyes together with Zaditen, wait at least 5 minutes between applying each product.
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This is particularly important for medicines which are used to treat:
• depression
• allergy (e.g. antihistamines)
Zaditen with food, drink and alcohol
Zaditen may increase the effect of alcohol.
Pregnancy and breast-feeding
If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. Zaditen can be used during breast-feeding.
Driving and using machines
Zaditen may cause blurred vision or drowsiness. If this happens to you, wait until this has cleared before driving or operating machinery.
How to use Zaditen
Always use this medicine exactly as your doctor or pharmacist have told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose for adults, elderly and children (age 3 and older) is one drop into the affected eye(s) twice a day (in the morning and evening).
Instructions for use
1. Wash your hands.
2. Open the bottle. Do not touch the tip after opening the bottle.
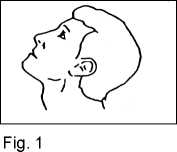
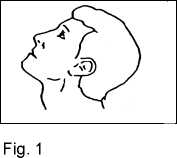
3. Lean your head back (Fig. 1).
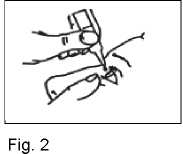
4. Pull down your lower eyelid with your finger and hold the bottle in your other hand.
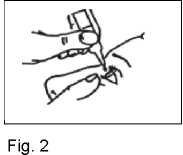
Squeeze the bottle so that one drop falls into the eye (Fig. 2).
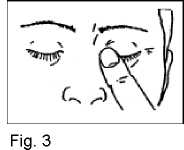
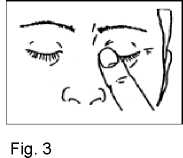
5. Close your eyes and press the tip of one finger against the corner of the eye for around 1-2 minutes. This will prevent the drop running through the tear duct into your throat and most of the drop will remain in the eye (Fig. 3). If necessary repeat steps 3 to 5 with your other eye.
6. Close the bottle after use.
If you have any further questions on the use of this product, ask your doctor or pharmacist or nurse.
If you use more Zaditen than you should
There is no danger if you have accidentally taken Zaditen by mouth or if you have used more than one drop in the eye. If you have any doubt contact your doctor for advice.
If you forget to use Zaditen
If you forget to use Zaditen you should treat your eyes as soon as you remember. Then continue with your normal routine.
Do not take a double dose to make up for a forgotten dose.
Q Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported.
Common (affecting less than 1 in every 10 patients)
• eye irritation or pain
• inflammation in the eye
Uncommon (affecting less than 1 in every 100 patients)
• blurred vision when putting drops on the eye
• dry eye
• eyelid disorder
• conjunctivitis
• increased sensitivity of the eyes to light
• visible bleeding in white of eye
• headache
• drowsiness
• rash (which may also itch)
• eczema (itchy, red, burning rash)
• dry mouth
• allergic reaction (including swelling of the face and eyelids) and increase in severity of existing allergic conditions such as asthma and eczema
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
Q Further Information What Zaditen contains
Each ml of eye drops contains 0.345mg ketotifen hydrogen fumarate corresponding to 0.25mg ketotifen as the active ingredient. The eye drops also contain benzalkonium chloride, glycerol (E422), sodium hydroxide (E524), water for injections.
What Zaditen looks like and contents of the pack
The eye drops are a clear, colourless to faintly yellow solution. They are available in bottles of 5ml.
Manufacturer and Licence Holder
Zaditen Eye Drops are manufactured by EXCELVISION, Rue de la Lombardiere, 07100 Annonay Cedex, France and is procured from within the EU and repackaged by the Product Licence Holder: Lexon (UK) Limited, Unit 18, Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 ORE.
POM PL 15184/0922 Leaflet revision date: 12/11/14
Blind or partially sighted?
Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
Q How to store Zaditen EXPIRY DATE
Do not use this medicine after date shown on the carton label or bottle label. Only keep this medicine if your doctor tells you to. If your eye drops becomes discoloured or shows any other signs of deterioration, consult your pharmacist (chemist) who will tell you what to do. This medicine must be thrown away four weeks after first opening the bottle.
HOW TO STORE
• Do not store above 25°C.
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Ref: 0922/121114/1 /B
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only.
Do not pass it on to others.It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Your medicine is called Ketotifen 0.25mg/ml Eye Drops Solution but will be referred to as Ketotifen throughout the leaflet.
What is in this leaflet:
What Ketotifen is and what it is used for What you need to know before you use Ketotifen
How to use Ketotifen
Possible side effects
How to store Ketotifen
Contents of the pack and other information
Ketotifen contains benzalkonium chloride
Ketotifen contains benzalkonium chloride and may cause eye irritation.
If you wear soft contact lenses you should remove
them before using Ketotifen as it can
discolour your soft contact lenses. You should wait
at least 15 minutes after using Ketotifen
before reinserting your contact lenses into your
eyes.
What Ketotifen is and what it is used for
Ketotifen contains the active substance ketotifen, which is an anti-allergic substance.
Ketotifen is used to treat eye symptoms of hay fever.
What you need to know before you use Ketotifen
Do not use Ketotifen
If you are allergic (hypersensitive) to ketotifen or any of the other ingredients of this medicine (listed in section 6).
Other medicines and Ketotifen
If you need to apply any other medicinal products to your eyes together with Ketotifen, wait at least 5 minutes between applying each product.
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This is particularly important for medicines which are used to treat:
• depression
• allergy (e.g. antihistamines)
Ketotifen with food, drink and alcohol
Ketotifen may increase the effect of alcohol.
Pregnancy and breast-feeding
If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. Ketotifen can be used during breast-feeding.
Driving and using machines
Ketotifen may cause blurred vision or drowsiness. If this happens to you, wait until this has cleared before driving or operating machinery.
How to use Ketotifen
Always use this medicine exactly as your doctor or pharmacist have told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose for adults, elderly and children (age 3 and older) is one drop into the affected eye(s) twice a day (in the morning and evening).
Instructions for use
1. Wash your hands.
2. Open the bottle. Do not touch the tip after opening the bottle.
3. Lean your head back (Fig. 1).
4. Pull down your lower eyelid with your finger and hold the bottle in your other hand.
Squeeze the bottle so that one drop falls into the eye (Fig. 2).
5. Close your eyes and press the tip of one finger against the corner of the eye for around 1-2 minutes. This will prevent the drop running through the tear duct into your throat and most of the drop will remain in the eye (Fig. 3). If necessary repeat steps 3 to 5 with your other eye.
6. Close the bottle after use.
If you have any further questions on the use of this product, ask your doctor or pharmacist or nurse
If you use more Ketotifen than you should
There is no danger if you have accidentally taken Ketotifen by mouth or if you have used more than one drop in the eye. If you have any doubt contact your doctor for advice.
If you forget to use Ketotifen
If you forget to use Ketotifen you should treat your eyes as soon as you remember. Then continue with your normal routine.
Do not take a double dose to make up for a forgotten dose.
Q Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported.
Common (affecting less than 1 in every 10 patients)
• eye irritation or pain
• inflammation in the eye
Uncommon (affecting less than 1 in every 100 patients)
• blurred vision when putting drops on the eye
• dry eye
• eyelid disorder
• conjunctivitis
• increased sensitivity of the eyes to light
• visible bleeding in white of eye
• headache
• drowsiness
• rash (which may also itch)
• eczema (itchy, red, burning rash)
• dry mouth
• allergic reaction (including swelling of the face and eyelids) and increase in severity of existing allergic conditions such as asthma and eczema
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
Q Further Information
What Ketotifen contains
Each ml of eye drops contains 0.345mg ketotifen hydrogen fumarate corresponding to 0.25mg ketotifen as the active ingredient. The eye drops also contain benzalkonium chloride, glycerol (E422), sodium hydroxide (E524), water for injections.
What Ketotifen looks like and contents of the pack
The eye drops are a clear, colourless to faintly yellow solution. They are available in bottles of 5ml.
Manufacturer and Licence Holder Ketotifen Eye Drops are manufactured by EXCELVISION, Rue de la Lombardiere, 07100 Annonay Cedex, France and is procured from within the EU and repackaged by the Product Licence Holder: Lexon (UK) Limited, Unit 18, Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 ORE.
POM PL 15184/0922 Leaflet revision date: 12/11/14
Blind or partially sighted?
Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
Q How to store Ketotifen EXPIRY DATE
Do not use this medicine after date shown on the carton label or bottle label. Only keep this medicine if your doctor tells you to. If your eye drops becomes discoloured or shows any other signs of deterioration, consult your pharmacist (chemist) who will tell you what to do. This medicine must be thrown away four weeks after first opening the bottle.
HOW TO STORE
• Do not store above 25°C.
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Ref: 0922/121114/2/B