Dorzolamide 20mg/Ml Eye Drops Solution
Read all of this leaflet carefully before you start
using this medicine because it contains important
information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Dorzolamide is and what it is used for
2. What you need to know before you use Dorzolamide
3. How to use Dorzolamide
4. Possible side effects
5. How to store Dorzolamide
6. Contents of the pack and other information
1. What Dorzolamide is and what it is used
Dorzolamide is a sterile eye drop solution. Dorzolamide contains dorzolamide, a sulphonamide-related compound, as the active ingredient.
Dorzolamide is an ophthalmic carbonic anhydrase inhibitor which reduces high pressure in the eye.
It is indicated in the treatment of elevated intra-ocular pressure in conditions such as ocular hypertension and glaucoma (open-angle glaucoma, pseudo-exfoliative glaucoma). Dorzolamide can be used alone or in addition to other medicines which lower the pressure in the eye (so-called beta-blockers).
2. What you need to know before you use Dorzolamide
Do not use Dorzolamide
- if you are allergic (hypersensitive) to dorzolamide or any of the other ingredients of this medicine (listed in section 6)
- if you have severe kidney problems or a prior history of kidney stones.
- if you have a disturbance in the pH (acid/alkali balance) of your blood.
Warnings and PrecautionsTalk to your doctor before using Dorzolamide
- if you have or have had liver problems in the past
- if you have been told you have a corneal defect
- if you have had, or are about to have eye surgery
- if you have suffered an eye injury or have an eye infection
- if you have had an allergic reaction including conjunctivitis or eyelid reaction
- if you have a prior history of kidney stones
- if you are taking another carbonic anhydrase inhibitor
- if you wear contact lenses (see the section ‘Important information about some of the ingredients of Dorzolamide).
Take special care with Dorzolamide
Tell your doctor about any medical problems you have now or have had in the past, including eye problems and eye surgeries, and about any allergies to any medications.
You should contact your doctor immediately if you develop any eye irritation or any new eye problems such as redness of the eye or swelling of the surface layer of the eye or eyelids.
Stop using Dorzolamide and contact your doctor immediately if you suspect that Dorzolamide is causing an allergic reaction (for example, skin rash or itching, inflammation of the eye).
Use in children and adolescents Dorzolamide has been studied in infants and children less than 6 years of age who have raised pressure in the eye(s) or have been diagnosed with glaucoma. For more information, talk to your doctor.
Use in elderly
In studies with Dorzolamide, the effects of Dorzolamide were similar in both elderly and younger patients.
Use in patients with liver impairment
Tell your doctor about any liver impairment problems you have now or have suffered.
Using other medicines
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
In particular you should tell your doctor if you are taking another carbonic anhydrase inhibitor such as acetazolamide, or a sulfa drug. You may be taking this type of medicine by mouth, as eye drops, or by some other method.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. Dorzolamide should not be used during pregnancy unless your doctor still recommends it.
Dorzolamide should not be used while breast-feeding.
Driving and using machines No studies on the effects on the ability to drive or use machines have been performed. There are side effects with this medicine, such as dizziness and blurred vision, which may affect your ability to drive and/or operate machinery. Do not drive or operate machinery until you feel well or your vision is clear.
Important information about some of the ingredients of Dorzolamide
Dorzolamide contains the preservative benzalkonium chloride
• Benzalkonium chloride may cause eye irritation
• Benzalkonium chloride is known to discolour soft contact lenses
• Avoid contact with soft contact lenses
• Remove contact lenses prior to application and wait until 15 minutes before reinsertion
3. How to use Dorzolamide
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The appropriate dosage and duration of treatment will be established by your doctor.
When Dorzolamide is used alone, the usual dose is one drop in the affected eye(s) three times a day, for example in the morning, in the afternoon and in the evening.
If your doctor has recommended you use Dorzolamide with a beta-blocker eye drop (medicines which lower the pressure of the eye), then the usual dose is one drop of Dorzolamide in the affected eye(s) two times a day, for example in the morning and in the evening.
If you use Dorzolamide with another eye drop, leave at least 10 minutes between putting in Dorzolamide and the other medicine. Alternatively if you are going to use Dorzolamide to replace another eye drop medicine, used to lower eye pressure, you should stop using the other medicine after taking the proper dosing on one day, and start Dorzolamide on the next day.
Do not change the dosage of the drug without consulting your doctor. If you must stop treatment, contact your doctor immediately.
Do not allow the tip of the container to touch your eye or areas around your eye. It may become contaminated with bacteria that can cause eye infections leading to serious damage of the eye, even loss of vision. To avoid possible contamination of the container, keep the tip of the container away from contact with any surface.
Instructions for proper use:
It is recommended that you wash your hands before putting in your eye drops.
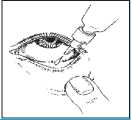
It may be easier to apply your eye drops in front of a mirror.
1. Before using the medication for the first time, be sure that the tamper-proof seal on the bottle neck is unbroken. A gap between the bottle and the cap is normal for an unopened bottle.
2. Take off the cap of the bottle.
3. Tilt your head back and gently pull your lower eyelid down to form a small pocket between your eyelid and your eye.
4. Invert the bottle and squeeze it until a single drop is dispensed into the eye as directed by your doctor. DO NOT TOUCH YOUR EYE OR EYELID WITH THE DROPPER TIP.
5. Repeat steps 2 & 3 with the other eye if instructed to do so by your doctor.
6. Put the cap back on and close the bottle straight after you have used it.
7. The dispenser tip is designed to provide a single drop; therefore, do NOT enlarge the hole of the dispenser tip.
8. After you have used all doses, there will be some medicine left in the bottle. You should not be concerned since the extra amount of medicine has been added and you will get the full amount of medicine that your doctor prescribed. Do not attempt to remove the excess medicine from the bottle.
If you use more Dorzolamide than you should
If you put too many drops in your eye or swallow any of the contents, you should contact your doctor immediately.
If you forget to use Dorzolamide
It is important to use Dorzolamide as prescribed by your doctor.
If you miss a dose, use it as soon as possible. However, if it is almost time for the next dose, skip the missed dose and go back to your regular dosing schedule.
Do not take a double dose to make up for a forgotten dose.
If you stop using Dorzolamide
Dorzolamide should be used every day to work properly. If you want to stop treatment, talk to your doctor first.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Gastrointestinal disorders:
Common: nausea, bitter taste
Rare: throat irritation, dry mouth
General disorders and administration site conditions: Common: asthenia/fatigue
Rare: Hypersensitivity: signs and symptoms
of local reactions (palpebral reactions) and systemic allergic reactions including swelling of the face, lips, tongue, and/or throat which may cause difficulty in breathing or swallowing, hives and itching, rash, shortness of breath and more rarely bronchospasm (contraction of the smooth muscle in the bronchi)
Nervous system disorders:
Common: Headache
Rare: dizziness, numbness/tingling sensation
Renal and urinary disorders:
Rare: formation of urinary calculi
Respiratory, thoracic, and mediastinal disorders:
Rare: bleeding from the nose
Skin and subcutaneous tissue disorders:
Rare: skin inflammation, severe skin
reactions Reporting of side effects If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the national reporting system listed in Yellow Card Scheme Website: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Dorzolamide
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The chance of having a side effect is described by the following categories:
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle label and the carton after EXP: The expiry date refers to the last day of that month. Keep the bottle in the outer carton in order to protect from light. Store below 30°C.
Dorzolamide should be used within 28 days after the bottle is first opened. Therefore, you must throw away the bottle 4 weeks after you first opened it, even if some solution is left. To help you remember, write down the date that you opened it in the space on the carton.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
|
Very common |
More than 1 user in 10 |
|
Common |
1 to 10 users in 100 |
|
Uncommon |
1 to 10 users in 1,000 |
|
Rare |
1 to 10 users in 10,000 |
The following side effects may be seen with Dorzolamide.
Eye disorders:
Very common: burning and stinging
Common: inflammation or swelling of the
surface layer of the eye(s) and possible inflammation of the eyelid(s) and/or skin around the eye(s), watering or itching of the eye(s), blurred vision, effects on the surface of the eye
Uncommon: inflammation of the middle layer of
the eye
Rare: swelling of the surface layer of the
eye(s), choroidal detachment which may be accompanied by visual changes/disturbances (following eye surgery), ocular hypotony, redness of the eye(s), eye pain, crusting of the eyelid(s), temporary shortsightedness (which stops when the medicine is discontinued)
What Dorzolamide contains
- The active substance is dorzolamide. Each ml contains 20 mg dorzolamide (as dorzolamide hydrochloride).
- The other ingredients are Mannitol, Hydroxyethyl Cellulose, Benzalkonium Chloride (as a preservative), Sodium Citrate, Sodium Hydroxide for pH adjustment and Water for injection.
What Dorzolamide looks like and contents of the pack
Dorzolamide is a sterile, isotonic, buffered, colourless, slightly viscous solution in a white opaque medium density polyethylene bottle with a sealed dropper tip and a two-piece cap assembly. Each bottle contains 5 mL of the eye drop solution.
Dorzolamide is available in packs containing 1 bottle, 3 bottles or 6 bottles.
Not all pack sizes may be marketed.
Marketing Authorisation Holder Glenmark Pharmaceuticals Europe Limited Laxmi House, 2B Draycott Avenue,
Kenton, Middlesex HA3 0BU United Kingdom
Manufacturer Pharmathen S.A.
6, Dervenakion str.,
153 51 Pallini Attiki,
Greece