Multibic 2 Mmol/L Potassium Solution For Haemofiltration
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
MULTIBIC 2 mmol/l potassium Solution for Haemofiltration
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
MultiBic 2 mmol/l potassium is provided in a two-compartment bag with 4.75 L of an alkaline hydrogen carbonate solution in one compartment and 0.25 L of an acidic electrolyte, glucose solution in the other compartment.
BEFORE MIXING: each 1000 mL solution contains:
Acidic electrolyte, glucose solution (small compartment)
2.982 g 4.410 g 2.033 g
20.00 g
22.00 g
40 mmol 30 mmol 10 mmol 122 mmol 111 mmol
Potassium chloride Calcium chloride dihydrate Magnesium chloride hexahydrate Glucose anhydrous as Glucose monohydrate
K+
Ca2+
Mg2+
Cl-
Glucose
Alkaline hydrogen carbonate solution (large compartment)
6.453 g 3.104 g
147.37 mmol 100.42 mmol 36.95 mmol
Sodium chloride Sodium hydrogen carbonate
Na+
Cl-
HCO3
AFTER MIXING:
1000 mL of the ready-to-use solution multiBic 2 mmol/l potassium contains:
Sodium chloride 6.136 g
Potassium chloride 0.1491 g
Sodium hydrogen carbonate 2.940 g
Calcium chloride dihydrate 0.2205 g
Magnesium chloride hexahydrate 0.1017 g
|
Glucose anhydrous |
1.000 |
g |
|
as Glucose monohydrate |
1.100 |
g |
|
Na+ |
140 |
mmol |
|
K+ |
2.0 |
mmol |
|
Ca2+ |
1.5 |
mmol |
|
Mg2+ |
0.50 |
mmol |
|
Cl- |
111 |
mmol |
|
HCO3- |
35 |
mmol |
|
Glucose |
5.55 |
mmol |
For a full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Solution for haemofiltration.
The Solution is clear and colourless.
Theoretical osmolarity: 296 mosm/l pH ~ 7.2
4. CLINICAL PARTICULARS
4.1 Therapeutic Indications
For use in patients with acute renal failure requiring continuous haemofiltration.
4.2 Posology and method of administration
Haemofiltration in patients with acute renal failure including the prescription of substitution solutions should be under the direction of a physician with experience in this treatment.
In acute renal failure treatment is carried out for a limited period and is discontinued when renal function is fully restored.
MultiBic 2 mmol/L potassium is exclusively indicated for intravenous infusion.
Infuse the ready-to-use solution into the extracorporeal circulation by means of a metering pump.
As blood serum is filtered off in haemofiltration the filtered volume, minus the necessary ultrafiltration fluid, must be substituted in the form of haemofiltration solution.
The filtration rate is prescribed by the attending physician depending on the clinical status and the body weight of the patient. Unless otherwise prescribed a total filtration rate of 800 to 1400 mL/h is appropriate in adults to remove metabolic waste products depending on the metabolic status of the patient. A maximum filtration rate of 75 L per day is recommended.
There is no clinical experience on the use and dosing of this product in children.
For instructions on use of the product, see section 6.6
4.3 Contraindications
Solution dependent contraindications:
• Hypokalaemia
• Metabolic alkalosis
Haemofiltration dependent contraindications due to the technical procedure itself:
• Renal failure with increased hypercatabolism in cases where uraemic symptoms can no longer be relieved by haemofiltration.
• Inadequate blood flow from vascular access.
• If there is a high risk of haemorrhage on account of systemic anticoagulation.
4.4 Special warnings and precautions for use
The haemofiltration solution should be warmed prior to infusion with appropriate equipment to approximately body temperature and must not be infused under any circumstances below room temperature.
The warming of this solution to approximately body temperature must be carefully controlled verifying that the solution is clear and without particles.
During application of multiBic in CRRT, white calcium carbonate precipitation has been observed in the tubing lines in rare cases, particularly close to the pump unit and the heating unit warming multiBic.
Precipitations particularly can occur if the temperature of the multiBic solution at the inlet of the pump unit is already higher than 25°C.
Therefore, the multiBic solution in the tubing lines should be closely visually inspected every 30 min during CRRT in order to ensure, that the solution in the tubing system is clear and free from precipitate.
Precipitations may occur also with substantial delay after start of treatment.
If precipitate is observed, multiBic solution and CRRT tubing lines must be replaced immediately and the patient carefully monitored.
The serum potassium concentration must be checked regularly before and during haemofiltration. The potassium status of the patient and its trend during haemofiltration must be considered. If hypokalaemia is present or tends to develop, supplementation of potassium and / or changing to a substitution solution with higher potassium concentration may be required.
If hyperkalaemia tends to develop, an increase in the filtration rate and / or changing to a substitution solution with a lower potassium concentration may be indicated as well as usual measures of intensive care medicine.
In addition, the following parameters should be monitored before and during haemofiltration: Serum sodium, serum calcium, serum magnesium, serum phosphate, serum glucose, acid-base status, levels of urea and creatinine, body weight and fluid balance (for the early recognition of hyper- and dehydration).
Prior to use the solution bag must be carefully inspected as described in detail in Section 6.6 “Special precautions for disposal and other handling”.
Do not use before mixing the two solutions
4.5 Interaction with other medicinal products and other forms of interaction
The correct dosing of substitutions solutions and strict monitoring of clinical
chemistry parameters and vital signs will avoid interactions with other drugs.
The following interactions are conceivable:
• Electrolyte substitutions, parenteral nutrition and other infusions usually given in intensive care medicine interact with the serum composition and the fluid status of the patient. This must be considered when prescribing haemofiltration treatment.
• Haemofiltration treatment may reduce the blood concentration of drugs, especially of drugs with a low protein binding capacity, with a small distribution volume, with a molecular weight below the cut-off of the haemofilter and of drugs adsorbed to the haemofilter. An appropriate revision of the dose of such drugs may be required.
• Toxic effects of digitalis may be masked by hyperkalaemia, hypermagnesaemia and hypocalcaemia. The correction of these electrolytes by haemofiltration may precipitate signs and symptoms of digitalis toxicity, e.g. cardiac arrhythmia.
4.6 Fertility, Pregnancy and lactation
At present no clinical experience is available. The bicarbonate-buffered substitution solution must only be used after assessment of the potential risks and benefits for the mother and child.
4.7 Effects on Ability to Drive and Use Machines
Not relevant.
4.8 Undesirable Effects
Adverse reactions, such as nausea, vomiting, muscle cramps, hypotension and hypertension, may result from the treatment mode itself or may be induced by the substitution solution.
In general, the tolerability of bicarbonate buffered haemofiltration solution is good. However, the following potential side effects of the treatment can be anticipated:
Hyper- or hypohydration, electrolyte disturbances (e.g., hypokalaemia), hypophosphataemia, hyperglycaemia and metabolic alkalosis.
4.9 Overdose
After use of recommended doses no reports of emergency situations have arisen; moreover, the administration of the solution can be discontinued at any time. If fluid balance is not accurately calculated and monitored, hyperhydration or dehydration may occur, with the resultant associated circulatory reactions. These may be manifest through changes in blood pressure, central venous pressure, heart rate and pulmonary arterial pressure.
In cases of hyperhydration congestive cardiac failure and/or pulmonary congestion may be induced.
In cases of hyperhydration, ultrafiltration should be increased, and the rate and volume of substitution solution infused reduced. In cases of marked dehydration, ultrafiltration should be decreased or discontinued, and the volume of substitution solution infused increased as appropriate.
Over treatment may result in disturbances of electrolyte concentrations and the acid-base-balance, e. g. an overdose of bicarbonate may occur if an inappropriate large volume of substitution solution is infused/administered. This could possibly lead to metabolic alkalosis, decrease of ionised calcium or tetany.
5.1 Pharmacodynamic properties
Pharmacotherapeutic group Group: Solution for haemofiltration ATC code: B05Z B - Haemofiltrates
Basic principles of Haemofiltration:
During continuous haemofiltration water and solutes such as uremic toxins, electrolytes, and bicarbonate are removed from the blood by ultrafiltration. The ultrafiltrate is replaced by a substitution solution (a solution for haemofiltration), with a balanced electrolyte and buffer composition.
The ready-to-use haemofiltration solution is a bicarbonate-buffered substitution solution for intravenous administration for the treatment of acute renal failure of any origin by continuous haemofiltration.
The electrolytes Na+, K+, Mg2+, Ca2+, Cl-, and bicarbonate are essential for the maintenance and correction of fluids and electrolyte homeostasis (blood volume, osmotic equilibrium, acid-base balance).
5.2 Pharmacokinetic Properties
The ready-to-use haemofiltration solution must only be administered intravenously.
The distribution of electrolytes and bicarbonate is regulated in accordance with requirements and the metabolic status and residual renal function. The active substances of the substitution solution are not metabolised except for glucose. The elimination of water and electrolytes depends on cellular requirements, the metabolic status, the residual renal function and on other routes of fluid losses (e.g., gut, lung, and skin).
5.3 Preclinical Safety Data
There are no preclinical data of relevance to the prescriber.
6. PHARMACEUTICAL PARTICULARS
6.1 List of Excipients
In small chamber A: Water for injections, Hydrochloric acid 25% In large chamber B: Water for injections, Carbon dioxide
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products. If an addition to the substitution solution is done, it should be done only after evaluating the compatibility with the substitution solution and only after the two chambers of the substitution solution have been thoroughly mixed.
6.3 Shelf life
Shelf life of the medicinal product packaged for sale 1 year
Shelf life after opening of the container: -
Shelf life after mixing: 48 hours
Chemical and physical in-use stability of the ready-to-use solution has been demonstrated for 48 hours at 25°C. Other in-use storage times and conditions prior to use (longer than 48 hours including the duration of the treatment, higher than 25°C prior to the inlet of the pump unit) are the responsibility of the user.
From a microbiological point of view, once connected to the haemofiltration circuit, and as hydrogen carbonate is present, the product shall be used immediately. Other in-use storage times and conditions are the responsibility of the user.
6.4 Special Precautions for Storage
Do not store below +4°C
6.5 Nature and contents of container
Double chamber bag:
4.75 l (alkaline hydrogen carbonate solution) + 0.25 l (acidic electrolyte, glucose solution) = 5.0 l (ready-to-use solution)
The film used for the bag is made of polyethylene terephthalate, which is coated with SiOx as a gas barrier, polyamide and a polypropylene-synthetic elastomer blend.
Overwrapping:
The double chamber bag is wrapped into a film made of a polyolefine-synthetic elastomer blend.
2 bags of 5000 ml (carton)
6.6 Special precautions for disposal
The haemofiltration solution should be administered in the following steps:
1. Removal of the overwrapping and careful inspection of the haemofiltration bag.
The overwrap should only be removed immediately before administration.
Plastic containers may occasionally be damaged during transport from the manufacturer to the dialysis clinic or within the clinic itself. This can lead to contamination and microbiological or fungal growth in the haemofiltration solution. Careful visual inspection of the container before connection and of the solution before use is therefore necessary. Particular attention should be paid to even the slightest damage to the closure, the welded seam and the corners of the container in view of a possible contamination.
The solution should only be used if clear and colourless and if the container and connectors are undamaged and intact.
In case of doubt, the treating physician should decide about the use of the haemofiltration solution.
2. Mixing of the two compartments
The two-compartment bag - the bicarbonate and the electrolytes including glucose compartments - are mixed immediately before use to obtain a solution ready-for-use. The mixed solution is clear and colourless.
After mixing both compartments, it must be checked, that the peel seam is completely open, that the solution is clear and colourless and that the container is not leaking.
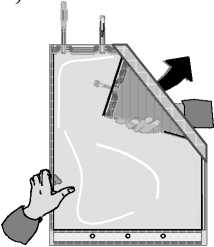
B)
Unfold the small compartment.
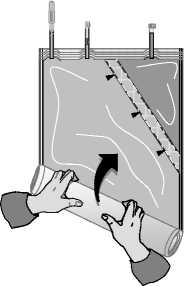
C)
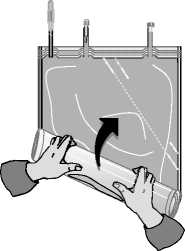
Roll up the solution bag starting from the corner opposite the small compartment ...
... until the peel seam between both compartments has opened along its entire length and the solutions from both compartments are mixed.
3. Ready-to-use solution
Any addition to the substitution solution should only be done after the substitution solution has been thoroughly mixed (see also 6.2). After such an addition, the substitution solution should again be thoroughly mixed prior to the start of the infusion.
The ready-to-use solution should be used immediately, but within a maximum of 24 hours after mixing.
If not otherwise prescribed, the ready-to-use substitution solution should be warmed immediately before infusion to 36.5 °C - 38.0 °C. The exact temperature must be selected depending on clinical requirements and the technical equipment used.
The haemofiltration solution is for single use.
Partially used and damaged containers should be discarded.
7. MARKETING AUTHORISATION HOLDER
Fresenius Medical Care Deutschland GmbH
D-61346 Bad Homburg
Germany
8. MARKETING AUTHORISATION NUMBER
PL 13689/0015
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE
AUTHORISATION
25/02/2009
10 DATE OF REVISION OF THE TEXT
26/04/2012