Nitromin 400 Mcg Per Actuation Sublingual Spray
<v
to
3
a>
<L>
-M CO
i_ 4-J
— *c
E
o
4> OO
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Nitromin Sublingual Spray (henceforth Nitromin Spray) is and what it is used for
2. What you need to know before you use Nitromin Spray
3. How to use Nitromin Spray
4. Possible side effects
5. How to store Nitromin Spray
6. Contents of the pack and other information
1. What Nitromin Spray is and what it is used for
Nitromin Spray is a smooth muscle relaxant. The walls of many of the body's veins and arteries are made of smooth muscle, and so glyceryl trinitrate helps to widen these vessels.
When you spray Nitromin Spray under your tongue it is quickly absorbed in the bloodstream. The action of Nitromin Spray allows blood to flow more quickly and more easily. This means that your heart does not need to work so hard. The greater blood flow through the heart also means that the heart works more efficiently. These actions of glyceryl trinitrate bring relief in an attack of angina and can prevent an attack coming on.
Nitromin Spray is for the relief and prevention of angina attacks (chest pains).
2. What you need to know before you use Nitromin Spray
Do not use Nitromin Spray if you:
Are allergic to glyceryl trinitrate, to other nitrates, or any of the other ingredients of this medicine (listed in section 6)
(you have previously had swelling of the face, extremities, lips, inside of the mouth, tongue, glottis and/or larynx while using any of the nitrate preparations)
Have marked hypotension (low blood pressure with a systolic pressure below 90mmHg)
Have too little circulating blood (due to blood loss or dehydration)
Suffer from conditions where your heart is unable to supply enough oxygenated blood to the rest of your body (cardiac shock or left sided heart failure)
Have angina pectoris caused by hypertrophic, obstructive cardiomyopathy (abnormal heart growth)
Have constrictive pericarditis
(an inflammation of the smooth membrane
that surrounds the heart)
Have pericardial tamponade
(an accumulation of fluid in the sac that
surrounds the heart)
Have aortic and mitral stenosis (heart valve disease)
Suffer from primary pulmonary hypertension (an increased resistance to blood flow through the lungs)
Have had a heart attack with low blood supply to the heart Are very anaemic
Take sildenafil, tadalafil or vardenafil (these drugs are used for the treatment of male impotence)
Suffer from any condition with possible raised pressure within your head including that caused by an accident
Warnings and precautions
Talk to your doctor or pharmacist before using Nitromin Spray
If you are prone to postural hypotension (low blood pressure) which can lead to dizziness and/or fainting on sitting up or standing up,
If you suffer from narrow angle glaucoma (increased pressure in the eye), or migraine,
If you suffer from liver disease, alcoholism, epilepsy, cerebral trauma and other nervous system diseases (the spray contains small amounts of alcohol and may be harmful to patients with the above conditions),
If you suffer from a disease of the blood vessels supplying the brain (cerebrovascular disease),
If you suffer from lung disease or lung disease related heart disease,
If you had a recent heart attack (myocardial infarction).
If your dose is increased, you may become tolerant to this medicine (where you need to use higher doses to prevent or relieve the symptoms of an angina attack) or to other medicines containing nitrates.
If this medicine does not work it may be an early sign of a myocardial infarction (heart attack). If this occurs, you should see your doctor immediately or go to the casualty department.
Please tell your doctor or nurse that you are using this medicine if you are having blood or urine tests, as it may affect the results.
Children
The use of Nitromin Spray is not recommended for children.
Other medicines and Nitromin Spray
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Never use Nitromin Spray with:
Sildenafil, vardenafil or tadalafil containing erectile dysfunction preparations.
The blood-pressure lowering effect of Nitromin Spray may be increased, possibly resulting in collapse, unconsciousness and even death.
Take care when using Nitromin Spray with: Medicines with blood pressure lowering effect, such as vasodilators and other antihypertensive agents, neuroleptics (used to treat mental illness), drugs used to treat depression (e.g. tricyclic antidepressants), sapropterin (used to treat phenylketonuria) and N-acetylcysteine (amino acid supplement). Any one of these medicines could increase the blood pressure lowering effect of glyceryl trinitrate. Dihydroergotamine containing preparations for the treatment of abnormal bleeding from the female reproductive system as the effect of this drug can be increased by glyceryl trinitrate.
Heparin containing blood-thinning preparations (the effectiveness of heparin may decrease).
You may need a higher dose of glyceryl trinitrate if you have been previously treated with long-acting nitrates (e.g. isosorbide dinitrate, isosorbide mononitrate).
If your doctor has prescribed tablets to be placed under the tongue for your angina, you should not use your spray for the same attack.
Nitromin Spray with alcohol
Consumption of alcoholic beverages during the use of the preparation is strictly forbidden as some of the side effects may be worse than usual (see section 4).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Nitromin Spray should only be used in women during pregnancy or breast-feeding, if the doctor considers that the benefit for the mother exceeds any possible risk to the baby.
Driving and using machines
It is not recommended to drive or use machinery when you start using this product as the hypotensive (blood pressure lowering) effect may lead to dizziness and/or feeling faint or unwell. After you have got used to the spray, it may be that you have no problems and are able to drive safely.
Nitromin Spray contains small amounts of ethanol (alcohol) and propylene glycol
This product contains 79.2 volume% of ethanol (alcohol). Each dose (spray) contains up to 0.04g of alcohol. This product may be harmful for patients suffering from liver disease, alcoholism, epilepsy, brain injury or disease.
Propylene glycol may cause skin irritation.
3. How to use Nitromin Spray
Always use this medicine exactly as your doctor or pharmacist have told you. Check with your doctor or pharmacist if you are not sure. You may be given Nitromin Spray in either a transparent plastic or aluminium canister.
If you have a transparent plastic canister follow the instructions below to take off the cap:
If you have a transparent plastic canister, when using for the first time, you need to take off a shirt collar safety ring. (see Figure 1.)
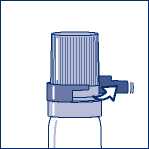
Figure 1. - Taking off shirt collar safety ring
Continued top of the next column
Continued over page
Then taking the transparent plastic canister in one hand, you can easily snap the cap off sideways with the other hand. (see Figure 2.)
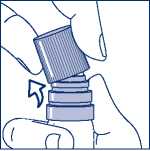
Figure 2. - Snapping off the cap
If you have an aluminium canister follow the instruction below to take off the cap:
When using the aluminium canister, you can easily snap the cap off (see Figure 3.)
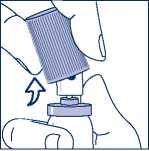
Figure 3. - Snapping off the cap without shirt collar safety ring from the aluminium canister
Before using the spray for the first time, check that the pump is working by pressing the pump button a few times until it produces a fine mist of liquid. Practice aiming the spray onto a tissue or similar item so that you will be able to aim it correctly under the tongue (sublingual use) when you need to use it. If you do not need to use the spray very often, remember to check the pump regularly to see that it still works properly.
If possible, sit down before using your spray.
Rest or sit quietly, as you may feel faint or dizzy otherwise, particularly if you are elderly. Take off the plastic cap and hold the spray bottle upright.
There is no need to shake the canister before dosing.
The spray outlet should then be placed close to the mouth. Hold your breath for a moment to avoid breathing in the spray, then open your mouth and spray a dose under your tongue by pressing down on the pump button then letting go. The mouth should be closed immediately after each dose (spray). The spray should not be breathed in.
It is useful to become aware of the position of the spray outlet so that its using at night can be easily achieved.
At the onset of an attack use one or two doses (sprays) under your tongue, and then immediately close your mouth before breathing normally through your nose again.
If symptoms do not resolve, this dose may be repeated at five minute intervals for a total of three doses (sprays).
If symptoms have not resolved after a total of three doses (sprays), seek prompt medical attention.
For the prevention of an attack use one or two doses (sprays) immediately before the event.
Put the cap back on the spray bottle.
If you are not sure how to use the spray, ask your pharmacist to show you.
If you use more Nitromin Spray than you should
If you use too many sprays some of the side effects (see below) may be worse than usual.
In this case you should see your doctor immediately.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some people are more sensitive to the effects of glyceryl trinitrate than others.
If you experience one of the following side effects, stop using the spray and consult your doctor immediately:
Allergic skin reactions or in some cases hypersensitivity reactions and exfoliative dermatitis (itchy, scaling skin rash) may appear.
Worsened angina pectoris symptoms (increase of chest pain). In cases where there is a large fall in blood pressure, this medicine may make the symptoms of angina worse (may affect up to 1 in 100 people).
Cyanosis (blue or purple coloration of the skin or mucous membranes due to the tissues near the skin surface being low on oxygen) (may affect up to 1 in 100 people). Methaemoglobinaemia (a blood disorder where oxygen cannot be released effectively to parts of your body) causes bluish skin colouring, headache, tiredness and lack of energy or shortness of breath (may affect up to 1 in 10,000 people).
Decreased blood flow to the brain (may affect up to 1 in 10,000 people).
The following side effects have also been reported: Very common (may affect more than 1 in 10 people)
Headache
Common (may affect up to 1 in 10 people) Dizziness Drowsiness Fast heart beat Decreased blood pressure*
Fall of blood pressure when standing up or sitting up*
Weakness
Uncommon (may affect up to 1 in 100 people) Syncope (loss of consciousness)
Slow heart beat Facial flushing
Circulatory collapse (failure of the blood circulation)
Nausea (feeling sick)
Vomiting (being sick)
Very rare (may affect up to 1 in 10,000 people) Restlessness Heartburn Bad breath Difficulty breathing Skin rash
* Particularly upon initiation of therapy and following an increase in dose. The first dose, or the first higher dose, may cause a blood pressure drop and/or postural hypotension (fainting or feeling faint when standing up or getting out of bed), tachycardia (your heart may seem to speed up), dizziness and weakness.
The following side effects may also occur in approximately 30-40% of patients: taste disturbance (metallic taste), mild burning or stinging sensation in the mouth or tongue, and palpitations (feeling your heartbeat).
These are usually mild and disappear within a few minutes.
Headache may occur at the beginning of the treatment.
Sometimes the heart rate may slow and even lead to feeling faint or actually fainting.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme (Website: www.mhra.gov.uk/yellowcard). By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Nitromin Spray
Store below 25°C, in the original package in order to protect from light.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the base of the aluminium canister or on the label of plastic canister.
Do not use it near to anyone that is smoking; or spray the product into a naked flame or onto a hot surface.
Do not pierce or burn the canister after use.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What Nitromin Spray contains
The active substance is glyceryl trinitrate 0.8% w/w (400 micrograms per spray)
The other ingredients are ethanol, propylene glycol and a particle free solution in a CFC-free spray.
What Nitromin Spray looks like and contents of the pack
The aluminium or transparent plastic canister is equipped with a metered dose valve and a protective cap. Each canister containing 10g of solution is designed to deliver at least 180 doses or 11g of solution is designed to deliver at least 200 doses.
Marketing Authorisation Holder
EGIS Pharmaceuticals UK Ltd.
127 Shirland Road London W9 2EP
United Kingdom
Manufacturer
EGIS Pharmaceuticals PLC Matyas Kiraly ut 65.
Kormend 2
H-9900
Hungary
This leaflet was last revised in March, 2016 PL 18190/0010
■o f-\
flj 1
n
dT £ era £2
a> *—
£U r-t
3
o
3
£U
r-t-
o'
3
—ti
O
—t
r-h
=T
O
c
to
(D
Continued top of the next column
Distributor Item