Nitromin 400 Mcg Per Actuation Sublingual Spray
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT
NITROMIN 400 micrograms per actuation sublingual spray
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each metered dose contains 400 micrograms of glyceryl trinitrate Excipients with known effect: ethanol, propylene glycol.
For the full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Sublingual spray
Colourless or almost colourless, clear solution, free from sediments, filled into a colourless, transparent, COC (cycloolefin copolymer) bottle for aerosols, equipped with a metered dose valve.
Colourless or almost colourless, clear solution, free from sediments, filled into an Al bottle, equipped with a metered dose valve.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
For the treatment and prophylaxis of angina pectoris and variant angina.
4.2 Posology and method of administration
Posology
Adults and the Elderly:
At the onset of an attack: one or two 400 microgram metered doses (sprays) should be sprayed under the tongue. If symptoms do not resolve, this dose may be repeated at five minute intervals for a total of three doses (sprays). If symptoms have not resolved after a total of three doses (sprays), the patient should seek prompt medical attention.
For the prevention of exercise-induced angina or in other precipitating situations, it is recommended to administer one or two 400 microgram metered doses (sprays) sprayed under the tongue immediately prior to the event.
Paediatric population
No data are available on the use of glyceryl trinitrate in children.
Nitromin 400 micrograms per actuation sublingual spray is not recommended for use in children.
Method of adminstration
Nitromin spray is marketed either in a transparent plastic or aluminium canister.
If you have a transparent plastic canister, when using for the first time, you need to take off a shirt collar safety ring. (see Figure 1.)
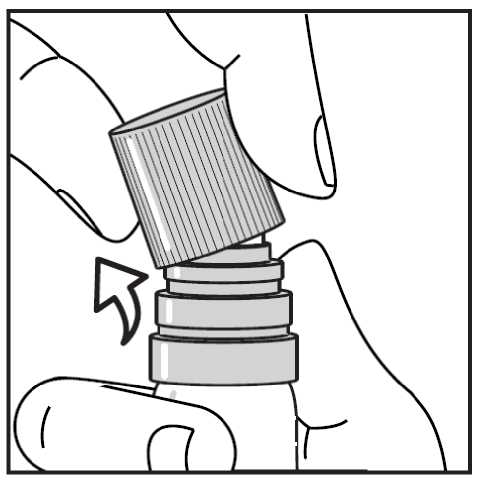
Then taking the transparent plastic canister in one hand, you can easily snap the cap off sideways with the other hand. (see Figure 2.)
When using the aluminium canister, you can easily snap the cap off. (see Figure 3.)
There is no need to shake the canister before dosing. The canister should be held vertically with the spray head uppermost. The pump may need to be primed when first used or if the product has not been used for a period of time. The priming actuation should be released into the air. The spray orifice should then be placed close to the mouth and the dose should be sprayed under the tongue. The mouth should be closed immediately after each dose. The spray should not be inhaled. Patients should be instructed to familiarize themselves with the position of the spray orifice so that administration at night can be easily achieved. During application the patient should preferably rest in the sitting position because of the risk of symptomatic postural hypotension. Hypotension and syncope can be a particular problem with use of nitrates in the elderly.
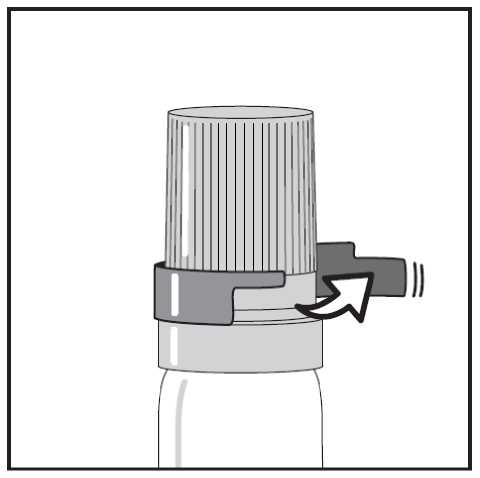
Figure 2. - Snapping off the cap
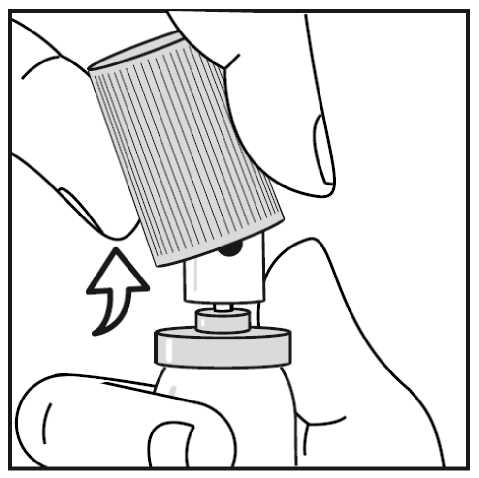
4.3 Contraindications
- Hypersensitivity to the active substance, to other nitro compounds, or to any of the excipients listed in section 6.1.
- Acute circulatory failure (shock, collapse).
- Hypovolaemia.
- Marked hypotension (systolic pressure below 90 mmHg).
- Cardiogenic shock (unless an appropriate left-ventricle end-diastolic pressure is ensured by intra-aortic balloon pump or by positive inotropic agents).
- Acute myocardial infarction with low filling pressure.
- Left heart failure with low filling pressure.
- Angina pectoris caused by hypertrophic, obstructive cardiomyopathy as it may exaggerate outflow obstruction.
- Constrictive pericarditis.
- Pericardial tamponade.
- Aortic and mitral stenosis.
- Primary pulmonary hypertension (since hyperaemia of hypoventilated alveolar regions may lead to hypoxia). Coronary patients are especially at risk in this respect.
- Possible increased intracranial pressure (e.g. cerebral haemorrhage or head trauma).
- Severe anaemia.
- Glyceryl trinitrate is contraindicated in patients taking phosphodiesterase type 5 inhibitors (e.g. sildenafil, vardenafil, tadalafil) (see section 4.5).
4.4 Special warnings and precautions for use
Glyceryl trinitrate should be used with caution in patients in whom adequate preload is important for maintaining cardiac output (e.g. orthostatic dysfunction) because administration of a vasodilator in these patients may worsen clinical status.
Glyceryl trinitrate should be used with caution in patients with cerebrovascular disease since symptoms may be precipitated by hypotension.
Glyceryl trinitrate may worsen hypoxaemia in patients with lung disease or cor pulmonale.
Arterial hypotension with bradycardia may occur in patients with myocardial infarction; this is thought to be reflexly mediated.
If angina symptoms have not resolved after a total of three doses, the patient should be instructed to seek prompt medical attention (see section 4.2).
Special caution and close medical control is required in patients predisposed to postural hypotension.
The preparation should be administered carefully to patients with narrow angle glaucoma, or migraine.
There is a great interindividual variation as regards the sensitivity of the patients to nitrates. This should always be kept in mind when setting the dosage.
Any increase of the dose may lead to tolerance.
Tolerance and crosstolerance to other nitrates may occur.
Glyceryl trinitrate increases the urinary excretion of catecholamines and VMA (vanillylmandelic acid).
The preparation contains 79.2 volume% of ethanol (alcohol). Each dose (spray) contains 0.0396g of alcohol. Its use may cause harm in liver disease, alcoholism, epilepsy, cerebral trauma and other CNS diseases, pregnancy and childhood. The preparation may modify or intensify the effect of other agents.
The preparation contains propylene glycol and may cause skin irritation.
Any lack of effect may be an indicator of early myocardial infarction.
4.5 Interaction with other medicinal products and other forms of interaction
Never co-administer with:
Consistent with its known effects on the nitric oxide/cyclic guanosine monophosphate (cGMP) pathway, phosphodiesterase type 5 inhibitors (e.g. sildenafil, vardenafil and tadalafil) have been shown to potentiate the hypotensive effects of nitrates, (possibly resulting in collapse, unconsciousness and even death) and coadministration with glyceryl trinitrate is therefore contraindicated (see section 4.3).
Consumption of alcoholic beverages during the use of the preparation is strictly forbidden.
Carefully combine with:
Treatment with other agents with hypotensive effects (e.g. vasodilators, antihypertensives, beta-blockers, calcium channel blockers and neuroleptics, tricyclic antidepressants and sapropterin) may potentiate the hypotensive effect of glyceryl trinitrate. In addition to these agents, the risk of hypotension and syncope with use of glyceryl trinitrate may be enhanced by alcohol.
N-acetylcysteine may potentiate the vasodilator effects of glyceryl trinitrate. Dihydroergotamine (the serum level and the effect of dihydroergotamine may increase);
There is evidence that systemic nitrates may interfere with the anticoagulant effects of heparin. Early and frequent monitoring of anticoagulation is recommended when systemic nitrates and heparin are used in combination. The efficacy of heparin may decrease.
The possibility of tolerance to the effects of glyceryl trinitrate should be considered when used in conjunction with long-acting nitrate preparations.
Patients with a history of previous nitrate treatment (with e.g. isosorbide-dinitrate, isosorbide-mononitrate) may require higher glyceryl trinitrate doses.
4.6 Fertility, pregnancy and lactation
Fertility
Animal studies did not indicate harmful effects with respect to fertility. However, the relevance of these animal findings to man is unknown. (See section 5.3).
Pregnancy
Animal studies did not indicate harmful effects with respect to pregnancy, embryofoetal development, parturition or postnatal development. However, the relevance of these animal findings to man is unknown. The administration of glyceryl trinitrate during pregnancy should only be considered if the expected benefit to the mother is greater than any possible risk to the foetus.
Breastfeeding
It is unknown if glyceryl trinitrate or its metabolites are excreted in human milk. A risk to the suckling child cannot be excluded. A decision must be made whether to discontinue/abstain from breast-feeding or to discontinue/abstain from glyceryl trinitrate therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman.
4.7 Effects on ability to drive and use machines
Since dizziness and syncope have been reported following treatment with glyceryl trinitrate, caution is recommended in patients performing skilled tasks.
4.8 Undesirable effects
Undesirable effects are listed below by system organ class and frequency. Frequencies are defined as follows: very common >1/10 (>10%); common >1/100 and <1/10 (>1% and <10%); uncommon >1/1000 and <1/100 (>0.1% and <1%); rare >1/10,000 and <1/1000 ( >0.01% and <0.1%); very rare <1/10,000 (< 0.01%).
|
Blood and lymphatic system disorders | |
|
Very rare |
Methaemoglobinaemia |
|
Psychiatric disorder | |
|
Very rare |
Restlessness |
|
Nervous system disorders | |
|
Very common |
Headache** |
|
Common |
Dizziness Drowsiness |
|
Uncommon |
Syncope |
|
Very rare |
Cerebral ischaemia |
|
Cardiac disorders |
|
Common |
Tachycardia |
|
Uncommon |
Enhanced angina pectoris symptoms*** Bradycardia Cyanosis |
|
Vascular disorders | |
|
Common |
Orthostatic hypotension* |
|
Uncommon |
Facial flushing Circulatory collapse |
|
Gastrointestinal disorders | |
|
Uncommon |
Nausea Vomiting |
|
Very Rare |
Heartburn Halitosis |
|
Respiratory, thoracic and mediastinal disorders | |
|
Very rare |
Impairment of respiration |
|
Skin and subcutaneous tissue disorders | |
|
Very rare |
Exfoliative dermatitis Drug rash |
|
General disorders and administration site conditions | |
|
Common |
Asthenia |
|
Investigations | |
|
Common |
Blood pressure decreased* |
|
Particularly upon initiation of therapy and fol |
owing an increase in dose. Occasionally, the |
first dose, or the first elevated dose may cause a blood pressure drop and/or postural hypotension with reflex tachycardia, dizziness and weakness.
Headache due to vasodilatation may occur at the beginning of the treatment.
***Sporadically, in cases with extreme blood pressure reduction, the treatment may aggravate the symptoms of angina pectoris (paradoxical nitrate reaction).
****Glyceryl trinitrate-induced hypotension may cause cerebral ischaemia.
The following side effects occur in approximately 30-40% of patients: taste disturbance (metallic taste), mild burning or stinging sensation in the mouth or tongue, and palpitations. These are usually mild and disappear within a few minutes.
In some cases hypersensitivity reactions, icluding allergic skin reactions may appear. Sometimes bradyarrhythmia occurs.
Sometimes collapse occurs.
Large dose of glyceryl trinitrate may cause vomiting, cyanosis, restlessness, methaemoglobinaemia and impairment of respiration.
During treatment with glyceryl trinitrate, temporary hypoxemia may occur due to a relative redistribution of the blood flow in hypoventilated alveolar areas.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme (Website: www.mhra.gov .uk/yellowcard).
Symptoms and Signs
Signs and symptoms encountered with overdose are generally similar to those events reported during treatment use although the magnitude and/or severity of the reactions may be more pronounced (see section 4.8). At very high doses an increase in intracranial pressure with cerebral symptoms may occur. Additional gastrointestinal effects such as colicky pain and diarrhoea have also been reported. Extreme doses cause methaemoglobinaemia, cyanosis, dyspnoea and tachypnoea.
Treatment
In the case of overdose, the patient’s clinical status including vital signs and mental status should be assessed and supportive treatment of the cardiovascular and respiratory systems provided as clinically indicated or as recommended by the national poisons centre, where available. The use of epinephrine (adrenaline) is contraindicated.
In the event of mild hypotension, passive elevation of the patient’s legs and/or lowering of the head may be effective.
Arterial blood gas estimation should be performed and if there is acidosis or the patient is clinically cyanosed, then severe methaemoglobinaemia must be assumed. Oxygen therapy should be given with 1 to 2 mg/kg bodyweight of i.v. Methylene Blue over five min unless the patient is known to have G-6-PD deficiency.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: vasodilators used in cardiac diseases, organic nitrates,
ATC Code: C01D A02
Glyceryl trinitrate is an organic nitrate compound acting as a vasodilator on both the arteries and the veins.
Mechanism of action:
Post-capillary capacity vessels, big arteries and especially coronary vessel sections that are still responsive are more sensitive to glyceryl trinitrate than the resistance vessels are. Vasodilatation in the systemic circulation increases venous capacity, and as a consequence decreases venous backflow to the heart (preload), ventricular volume and the filling pressure. All these events decrease the energy and oxygen demand of the myocardium. The reduced filling pressure improves the blood supply of subendocardial wall layers endangered by ischaemia, whereby regional wall movement and stroke volume are both improved. The dilatation of the big arteries near to the heart decreases both the systemic and the pulmonary vascular resistance. Glyceryl trinitrate exerts muscle relaxant effects also on the smooth muscle elements of bronchi, urinary tract, gallbladder, biliary tract, oesophagus, small and large intestines and sphincters.
Presumably, glyceryl trinitrate exerts its effects through binding to the so-called nitrate receptors located on the membrane of smooth muscle cells and inducing NO production and intracellular cGMP accumulation. By preventing calcium-ions from entering the cell, the accumulation of cGMP results in the relaxation of smooth muscle elements.
5.2 Pharmacokinetic properties
Upon sublingual administration, glyceryl trinitrate is rapidly absorbed from the oral cavity and the compound reaches the circulation without passing through the liver first. Bioavailability shows high inter- and intra-individual variations and amounts to 39 %, on the average. Glyceryl trinitrate has a rapid onset of action; the effect develops within 1-1.5 minutes and lasts for about 30 minutes. Maximum plasma level is attained within about 4 minutes. In the case of sublingual administration the plasma half-life is about 2.5-4.4 minutes. Circulating glyceryl trinitrate is strongly bound to the red blood cells and it is accumulated in the vascular wall. The binding to plasma proteins is about 60 %. The primary route of elimination is urinary excretion of metabolites; less than 1 % of the administered dose is excreted as unchanged compound.
5.3 Preclinical safety data
Long-term, high-dose oral treatment of mice with glyceryl trinitrate did not show any carcinogenic effect, however, similar treatment of rats increased the incidence of fibrotic or tumorous hepatic alterations.
High-dose reproduction toxicity tests demonstrated lower fertility in the offsprings of mice, however, the same study failed to show any teratogenic effect.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Propylene glycol Ethanol
Particle-free solution in a CFC-free spray.
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
Opened and unopened: 36 months
6.4 Special precautions for storage
Store below 25°C, in the original package in order to protect from light and radiant heat!.
The product is flammable, explosive. Storage and application near open fire and while smoking are forbidden.
Do not use it near to anyone that is smoking; or spray the product into a naked flame or onto a hot surface.
Do not pierce or burn the canister after use.
6.5 Nature and contents of container
The aluminium or transparent plastic canister (cycloolefin copolymer) are equipped with a metered dosing valve and a protective cap. Each 180 dose canister contains 10 grams of solution. Each 200 dose canister contains 11 grams of solution.
6.6 Special precautions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
The empty canister should never be pierced or thrown into fire.
7 MARKETING AUTHORISATION HOLDER
EGIS Pharmaceuticals U.K. Ltd.
127 Shirland Road London W9 2EP United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
PL 18190/0010
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE
AUTHORISATION
Date of first authorisation: 21 May 2003 Date of latest renewal: 03 March 2009
10 DATE OF REVISION OF THE TEXT
27/11/2014