Willfact 2000 I.U Powder And Solvent For Solution For Injection
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
WILLFACT 2000 IU
Powder and solvent for solution for injection
Human von Willebrand factor
Read all of this leaflet carefully before you start using this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Willfact is and what it is used for
2. Before you use Willfact
3. How to use Willfact
4. Possible side effects
5. How to store Willfact
6. Further information
1. WHAT WILLFACT IS AND WHAT IT IS USED FOR
Willfact is a medicine used to stop bleeding that contains human von Willebrand factor (VWF) as active ingredient.
Willfact is indicated in the prevention and treatment of surgical or other bleeding in patients with von Willebrand disease when desmopressin (DDAVP) treatment alone is ineffective or contraindicated.
2. BEFORE YOU USE WILLFACT Contraindications
Do not use Willfact
• if you are hypersensitive (allergic) to human von Willebrand factor or to one of the ingredients of Willfact.
• if you suffer from haemophilia A.
Take special care with Willfact:
Your treatment with Willfact should always be supervised by a physician experienced in the treatment of haemostatic disorders.
If you experience heavy bleeding and a blood examination shows that your Factor VIII blood value is reduced, you will receive the VWF preparation in addition to a Factor VIII preparation within the first twelve hours.
Allergic reactions
As with every protein medicine for intravenous use derived from human blood or plasma, hypersensitivity reactions in the form of an allergy may occur. During your injection, you will be observed specifically to determine whether you experience any early signs of hypersensitivity, e.g. stinging, hives (generalised urticaria), tightness of the chest, wheezing, drop in blood pressure (hypotension) and allergic severe reactions (anaphylaxis). If these symptoms occur, the injection will be interrupted immediately.
Risk of thrombosis
Blood vessels may also become blocked by blood clots (thromboses). This risk exists particularly if your previous medical history or laboratory results indicate that you present certain risk factors. In this case you will be monitored very carefully for the early signs of thrombosis, and a preventative treatment (prophylaxis) against vein blockages by blood clots should be introduced.
When using a Factor VIII-containing VWF product, your physician should be aware that the treatment may cause an excessive rise in FVIII:C. If you receive such FVIII-containing VWF product, your physician should monitor your FVIII:C plasma level regularly. This ensures that your FVIII:C plasma level is not sustained excessive, which may increase the risk of thrombotic events.
Limited effectiveness
It is possible that, in patients with von Willebrand disease, especially type 3 patients, proteins may be formed that neutralise the effect of VWF. These proteins are called antibodies or inhibitors. If the laboratory results give corresponding indications, or if the bleeding does not stop despite a sufficient dose of Willfact, your physician will check whether VWF inhibitors are being formed in your body. If these inhibitors are present in high concentration, treatment with VWF may not be effective, and other treatment options should be considered. The new treatment will be provided by a physician who has experience in the treatment of haemostatic disorders.
Safety of the raw material in Willfact (plasma)
The use of medicines derived from human blood or plasma is by definition associated with the risk of infections. Various standard measures are taken to counter this, including the targeted selection of donors, screening of individual donations and plasma pools for specific markers of infection and the inclusion of effective manufacturing steps for the inactivation/removal of viruses. Despite this, the possibility of transmitting of infective agents cannot be totally excluded when medicinal products prepared from human blood or plasma are administered. This also applies to unknown or emerging viruses and other pathogens.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus (HBV) or hepatitis C virus (HCV). The measures taken may be of limited value against non-enveloped viruses such as hepatitis A virus and parvovirus B19. Parvovirus B19 infection may be serious for pregnant women (foetal infection) and for individuals with immunodeficiency or certain forms of anaemia.
Vaccinations
If you are treated regularly with human plasma-derived von Willebrand factor, appropriate vaccinations (hepatitis A and hepatitis B) are recommended.
Recording of batch number
Every time Willfact is administered, the physician or one of his/her employees will record the name and batch number of the product in order to be able if necessary to trace precisely which batch(s) you have received. The batch number indicates the production run in which the batch of product in question was produced.
Using other medicines
Please tell your doctor or pharmacist if you are taking/using or have recently taken/used any other medicines, including medicines obtained without a prescription.
Using Willfact with food and drink
There are no known interactions of VWF preparations with foods or drinks. Therefore you do not have to avoid any specific foods or drinks.
Pregnancy and breast-feeding
Willfact should be used during pregnancy and breastfeeding only if it is clearly indicated. The safety of Willfact during pregnancy and breastfeeding has not been evaluated in controlled clinical studies. Animal studies are not sufficient to establish its safety with respect to fertility, pregnancy and development of the child during pregnancy and after birth.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
No effects on ability to drive or use machines have been observed.
Important information about some of the ingredients of Willfact
Willfact contains 13.8 mg of sodium per vial.
You should take it into consideration if you are on a salt-free or low-salt diet.
3. HOW TO USE WILLFACT
Treatment should only be initiated under the supervision of a physician experienced in the treatment of haemostatic disorders.
Dosage
The dose you take depends on your health condition and body weight.
The first dose of Willfact is 40 to 80 IU/kg for the treatment of haemorrhage or trauma, in conjunction with the required amount of factor VIII product, calculated according to your baseline plasma level of FVIII:C, in order to achieve an appropriate plasma level of FVIII:C, immediately before the intervention or as soon as possible after the onset of the bleeding episode or severe trauma.
If required, you will receive further doses of Willfact of 40 to 80 IU/kg per day in one or two injections daily over one to several days.
Willfact can also be administered as long-term prophylaxis; the dose level is also determined individually in this case. Willfact doses between 40 and 60 IU/kg administered two to three times per week reduce the number of haemorrhagic episodes.
Please talk to your doctor if you feel that the effect of Willfact is too strong or too weak.
If you use more Willfact than you should:
No symptoms of overdose with Willfact have been reported.
However, the risk of thrombosis cannot be excluded in case of major overdose.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Willfact can cause side effects, although not everybody gets them.
The assessment of side-effects is based on the following frequency specifications:
Very common: Affects more than 1 user in 10.
Common: Affects 1 to 10 users in 100.
Uncommon: Affects 1 to 10 users in 1,000.
Rare: Affects 1 to 10 users in 10,000.
Very rare: Affects less than 1 user 10,000.
Not known: Frequency cannot be estimated from the available data.
Although the safety of Willfact is considered good, allergic or anaphylactic reactions could occur.
The following side-effects occurred “uncommonly”:
• Accelerated heartbeat (tachycardia)
• Tightness of the chest
• Headache
• Restlessness
• Tingling
• Wheezing
• Nausea
• Vomiting
• Quincke’s oedema (angiooedema)
• Nettle rash (generalised urticaria)
• Hives
• Drop in blood pressure (hypotension)
• Burning and stinging at the infusion site
• Chills
• Flushing/heat
• Hypersensitivity - or allergic reactions
In some cases, the aforementioned signs may progress to severe allergic reaction (anaphylaxis) including shock.
• Listlessness (lethargy)
The following side-effects were observed “rarely”:
• Fever
Antibodies (inhibitors) against VWF: very rarely, proteins may be formed in patients with von Willebrand disease, especially type 3 patients, which neutralise the effect of VWF. These proteins are called antibodies or inhibitors. However, this has never been observed during Willfact treatment. Patients treated with VWF should be carefully monitored by their doctors for the development of inhibitors by appropriate clinical observations and laboratory tests. If such inhibitors occur, the condition will manifest itself as an inadequate clinical response. The antibodies form antibody-antigen complexes and occur concomitantly to anaphylactic reactions.
After correction of the factor Willebrand deficiency, you must be monitored for early signs of thrombosis or disseminated intravascular coagulation and receive treatment to prevent thrombosis in situations involving an increased risk of thrombosis (after operations, during confinement to bed, in cases of deficiency in a coagulation inhibitor or fibrinolytic enzyme).
If you receive FVIII-containing VWF preparations, the risk of thrombosis may also be increased due to persistently elevated FVIII:C plasma levels.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE WILLFACT
• Store in the original package in order to protect from light. Do not store above 25°C. Do not freeze.
• The product should be used immediately after reconstitution. However, its stability has been demonstrated for 24 hours at 25°C.
• Keep product out of the reach and sight of children.
• Do not use the product after the expiry date stated on the vial label and carton.
• Do not use Willfact if you notice that the solution is cloudy or that it contains any deposit.
Any unused product or waste material should be disposed of in accordance with local regulations.
6. FURTHER INFORMATION What Willfact contains
The active substance is: human von Willebrand factor (2000 IU/vial), expressed in International Units (IU) of Ristocetin Cofactor activity (VWF:RCo). After reconstitution with 20 ml of water for injections, one vial contains 100 IU human von Willebrand factor per 1 ml.
Before the addition of albumin, the specific activity is greater than or equal to 50 IU of VWF:RCo/mg of total protein.
The other ingredients are:
Powder: human albumin, arginine hydrochloride, glycine, sodium citrate and calcium chloride dihydrate.
Solvent: water for injections.
What Willfact looks like and contents of the pack
Willfact is presented as powder and solvent for solution for injection (2000 IU/20 ml).
One pack of Willfact contains one vial with 2000 IU human von Willebrand factor and one vial with 20 ml water for injections for reconstitution with a transfer system.
Marketing Authorisation Holder and Manufacturer
LFB-BIOMEDICAMENTS 3, avenue des Tropiques,
BP 305 - LES ULIS,
91958 Courtabmuf Cedex FRANCE
This medicinal product is authorised in the Member States of the EEA under the following names:
Austria Willfact 100 I.E./ml Pulver und Losungsmittel zur Herstellung einer
Injektionslosung Czech Republic Willfact
Denmark Willfact
Estonia Willfact
Germany WILLFACT 2000 I.E.
Hungary Willfact
Latvia Willfact
Lithuania Willfact 100 TV/ ml, milteliai ir tirpiklis injekciniam tirpalui
Norway Willfact
Poland Willfact
Portugal Willfact
Slovenia Willefact 2000 i.e Prasek in vehikel za raztopino za injiciranje
Slovak Republic Willfact
Spain Willfact
Sweden Willfact
United Kingdom Willfact
This leaflet was last approved in {12/2014}.
<..............................................................................................................>
The following information is intended for medical or healthcare professionals only: Reconstitution:
The currently applicable guidelines for aseptic procedures must be followed. The transfer system is only used to reconstitute the drug, as described below. It is not intended in administering the drug to the patient.


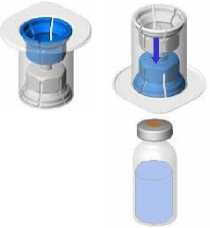



Bring the two vials (powder and solvent) to ambient temperature (not above 25°C).
Remove the protective cap from the solvent vial (water for injections) and from the powder vial.
Disinfect the surface of each stopper.
Remove the cap from the Mix2Vial device. Without removing the device from its packaging, attach the blue end of the Mix2Vial to the stopper of the solvent vial.
Remove and discard the packaging. Take care not to touch the newly-exposed part of the device.
Turn the solvent vial-device assembly over and attach to the powder vial using the transparent part of the device. The solvent will automatically transfer to the powder vial. Hold the assembly and gently swirl to completely dissolve the product.

The solution should be clear or slightly opalescent, colourless or slightly yellowish. Cloudy solutions or solutions with deposits must not be used.
Administration:

Hold the vial of reconstituted product in vertical position while screwing a sterile syringe onto the Mix2Vial device. Then slowly draw the product up into the syringe.
Once the product has been transferred to the syringe, firmly hold the syringe (with the piston pointing downward), unscrew the Mix2Vial device and replace it with an intravenous or butterfly needle.
Expel the air from the syringe and insert into the vein after disinfecting the surface.
Inject slowly by intravenous route immediately after reconstitution as a single dose at a maximum rate of 4 ml/minute.
Any unused product or waste material should be disposed of in accordance with local requirements.